Atoms a closer look at elements As mentioned











- Slides: 17

Atoms – a closer look at elements As mentioned before, atoms are composed of protons, neutrons, and electrons

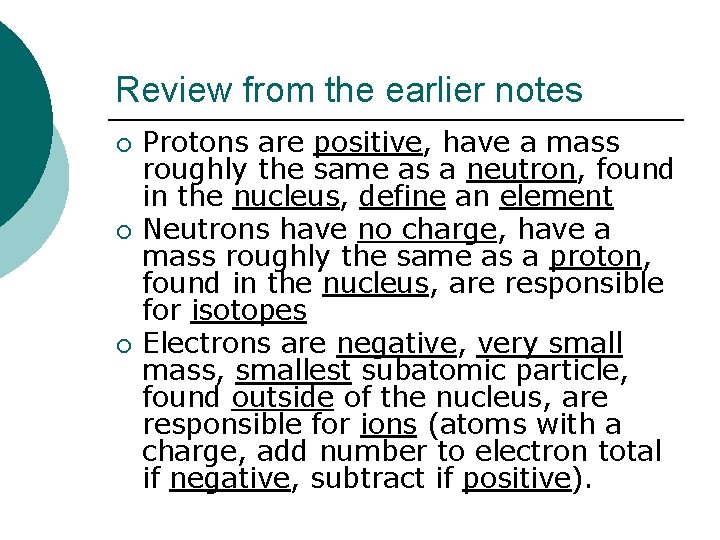
Review from the earlier notes ¡ ¡ ¡ Protons are positive, have a mass roughly the same as a neutron, found in the nucleus, define an element Neutrons have no charge, have a mass roughly the same as a proton, found in the nucleus, are responsible for isotopes Electrons are negative, very small mass, smallest subatomic particle, found outside of the nucleus, are responsible for ions (atoms with a charge, add number to electron total if negative, subtract if positive).

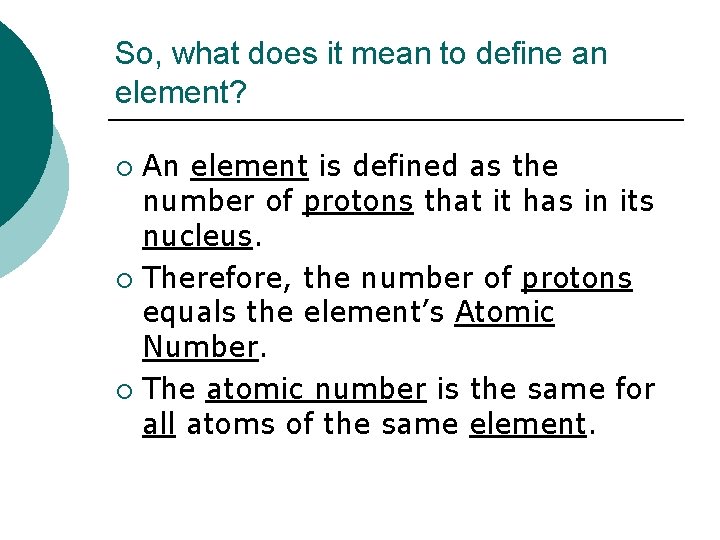
So, what does it mean to define an element? An element is defined as the number of protons that it has in its nucleus. ¡ Therefore, the number of protons equals the element’s Atomic Number. ¡ The atomic number is the same for all atoms of the same element. ¡

More about the Atomic number ¡ ¡ Equals the number of electrons for NEUTRAL atom Determines an elements placement on the periodic table Represented by letter Z So Z = protons = electrons (if neutral, if not see above note)

Let’s look at a Periodic Table How many protons does titanium (Ti) have? ¡ Antimony has 51 protons, what is its atomic number? What abbreviation is given to Antimony? ¡ Magnesium (Mg) is a neutral atom, how many electrons does it have? ¡

Mass number (A) Sum of the protons and neutrons of an atom ¡ So, Mass number = protons + neutrons ¡ To find neutrons given protons and mass number: ¡ l Neutrons = mass number - protons 108 47 Ag Top is mass number Bottom atomic number Note: Both numbers on left This is called a nuclear symbol

Practice Problem ¡ What is the mass number of an element with 10 protons and 10 neutrons? What element are we dealing with? How would you represent this symbolically? l l 20 amu Ne 20 l 10 Ne

More Practice ¡ ¡ ¡ How many protons, neutrons and electrons does the following neutral atoms have? 56 26 26 protons, 30 neutrons, 26 electrons ¡ 131 54 ¡ Fe Xe 54 protons, 77 neutrons, 54 electrons

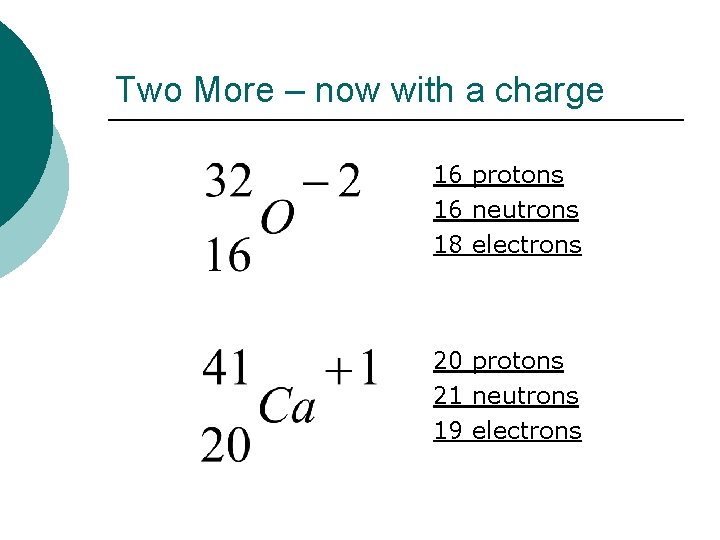
Two More – now with a charge 16 protons 16 neutrons 18 electrons 20 protons 21 neutrons 19 electrons

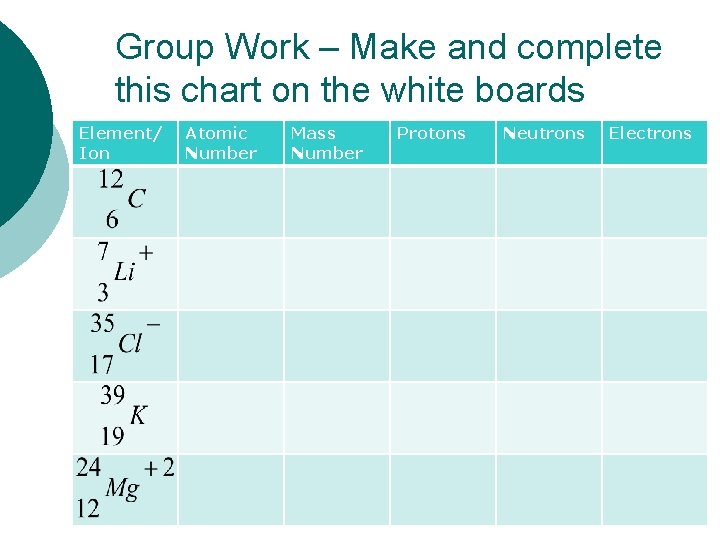
Group Work – Make and complete this chart on the white boards Element/ Ion Atomic Number Mass Number Protons Neutrons Electrons

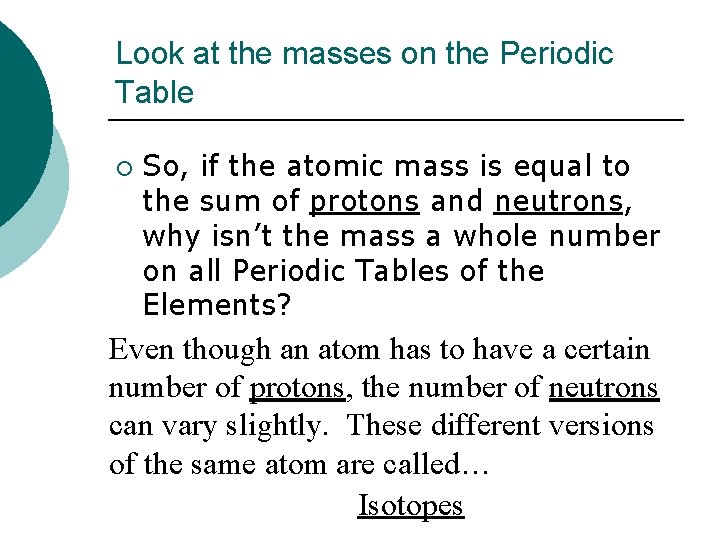
Look at the masses on the Periodic Table ¡ So, if the atomic mass is equal to the sum of protons and neutrons, why isn’t the mass a whole number on all Periodic Tables of the Elements? Even though an atom has to have a certain number of protons, the number of neutrons can vary slightly. These different versions of the same atom are called… Isotopes

Isotopes Same number of protons but different number of neutrons ¡ Examples are Carbon -13 verse Carbon -12 ¡

Average Atomic Masses Relative atomic mass: average masses of isotopes average atomic mass is also known as Atomic weight (AW). Atomic weights are listed on the periodic table. *Don’t confuse these with mass numbers*

Naturally occurring Carbon is 98. 892 % 12 C + 1. 108 % 13 C. Find the average atomic mass Step 1: Multiply the mass number of each isotope by its percentage ¡ Step 2: Add the numbers together ¡ Step 3: Divide by 100 ¡ Step 4: Check your answer. Mass should be between highest and lowest mass ¡

An Isotope Example - Chlorine Let’s look at Chlorine, which has an atomic mass of 35. 5 Atomic # = ___, 17 which means that there are ___ 17 protons. There are two naturally occurring isotopes, Cl-35 and Cl-37 This means that Cl-35 has ___ 18 neutrons while Cl-37 has ___ 20 neutrons. Cl-35 occurs naturally 75. 8% of the time Cl-37 occurs naturally 24. 2% of the time (35 x 75. 8 + 37 x 24. 2)/100 = 35. 5

Try this one Calculate the average mass of the isotopes of Uranium with: ¡ 50. 0% at 239 amu ¡ 29. 4% at 235 amu ¡ 20. 6% at 238 amu ¡ ¡ 237. 62 amu

Homework Page 108: 30 -37 ¡ Page 253: 43 and 44 ¡