A 540 Stellar Atmospheres Organizational Details Meeting times






- Slides: 14

A 540 – Stellar Atmospheres Organizational Details • • • Meeting times Textbook Syllabus Projects Homework • Topical Presentations • Exams • Grading • Notes

Basic Outline • Textbook Topics – Chapter 1 – Review of relevant basic physics – Chapter 5 – Radiation – Chapter 6 – Black bodies – Chapter 7 – Energy transport – Chapter 8 – Continuous Opacity – Chapter 9 – Model Photospheres – Chapter 10 – Stellar Continua – Chapter 11 – Line Absorption – Chapter 13 – Spectral Lines – Chapter 14 – Chemical Analysis – Chapter 15 – Radii and Temperatures • Additional Topics – Stars in the astrophysical zoo – Stellar rotation – Stellar activity – Winds and mass loss – White dwarf spectra and atmospheres – M, L and T dwarfs – Non LTE – Metal poor stars – Pulsating stars – Asteroseismology – Supergiants – Wolf-Rayet stars – AGB stars – Post-AGB stars – Peculiar A stars – Pre main sequence stars – Other ideas…

Goals • Familiarity with basic terms and definitions • Physical insight for conditions, parameters, phenomena in stellar atmospheres • Appreciation of historical and current problems and future directions in stellar atmospheres

History of Stellar Atmospheres • Cecelia Payne Gaposchkin wrote the first Ph. D thesis in astronomy at Harvard • She performed the first analysis of the composition of the Sun (she was mostly right, except for hydrogen). • What method did she use? • Note limited availability of atomic data in the 1920’s

Useful References • Astrophysical Quantities • Holweger & Mueller 1974, Solar Physics, 39, 19 – Standard Model • MARCS model grid (Bell et al. , A&AS, 1976, 23, 37) • Kurucz (1979) models – Ap. J Suppl. , 40, 1 • Solar composition – "THE SOLAR CHEMICAL COMPOSITION " by Asplund, Grevesse & Sauval in "Cosmic abundances as records of stellar evolution and nucleosynthesis", eds. F. N. Bash & T. G. Barnes, ASP conf. series, in press: see also Grevesse & Sauval 1998, Space Science Reviews, 85, 161 or Anders & Grevesse 1989, Geochem. & Cosmochim. Acta, 53, 197 • Solar gf values – Thevenin 1989 (A&AS, 77, 137) and 1990 (A&AS, 82, 179)

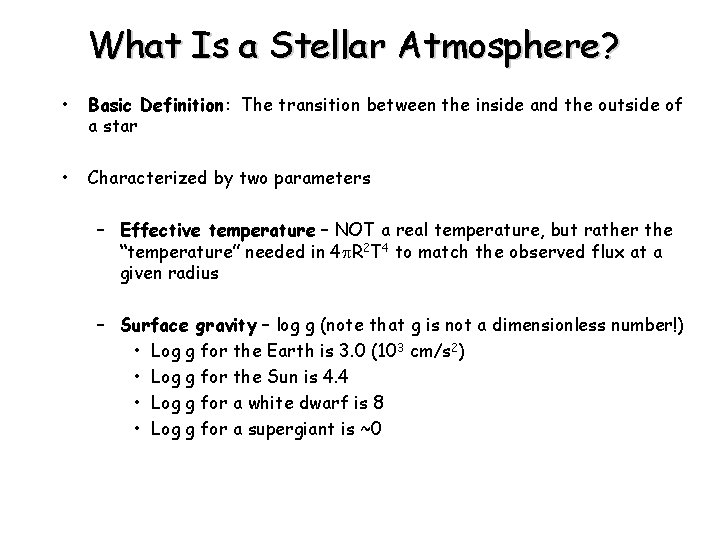
What Is a Stellar Atmosphere? • Basic Definition: The transition between the inside and the outside of a star • Characterized by two parameters – Effective temperature – NOT a real temperature, but rather the “temperature” needed in 4 p. R 2 T 4 to match the observed flux at a given radius – Surface gravity – log g (note that g is not a dimensionless number!) • Log g for the Earth is 3. 0 (103 cm/s 2) • Log g for the Sun is 4. 4 • Log g for a white dwarf is 8 • Log g for a supergiant is ~0

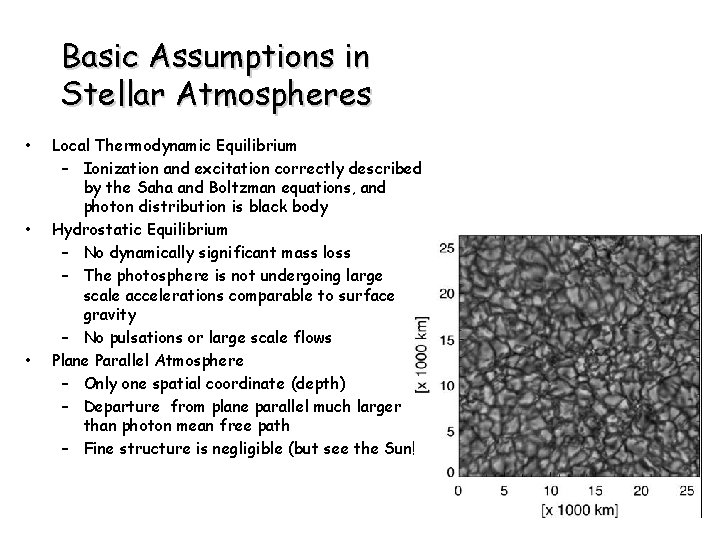
Basic Assumptions in Stellar Atmospheres • • • Local Thermodynamic Equilibrium – Ionization and excitation correctly described by the Saha and Boltzman equations, and photon distribution is black body Hydrostatic Equilibrium – No dynamically significant mass loss – The photosphere is not undergoing large scale accelerations comparable to surface gravity – No pulsations or large scale flows Plane Parallel Atmosphere – Only one spatial coordinate (depth) – Departure from plane parallel much larger than photon mean free path – Fine structure is negligible (but see the Sun!)

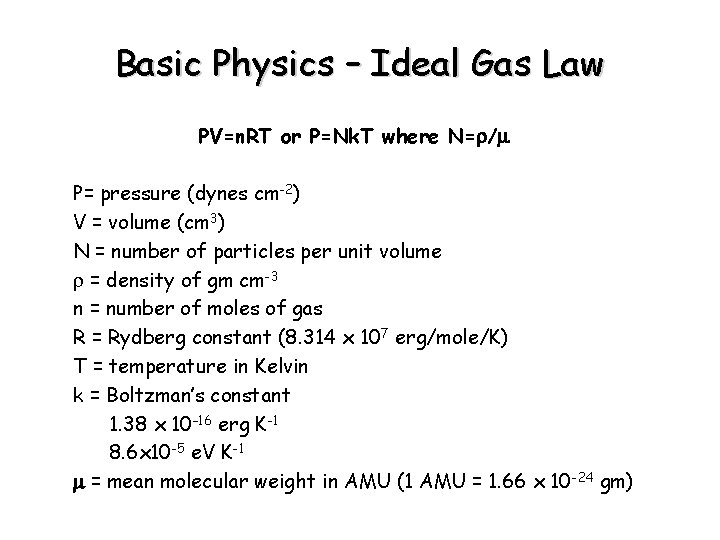
Basic Physics – Ideal Gas Law PV=n. RT or P=Nk. T where N=r/m P= pressure (dynes cm-2) V = volume (cm 3) N = number of particles per unit volume r = density of gm cm-3 n = number of moles of gas R = Rydberg constant (8. 314 x 107 erg/mole/K) T = temperature in Kelvin k = Boltzman’s constant 1. 38 x 10– 16 erg K-1 8. 6 x 10 -5 e. V K-1 m = mean molecular weight in AMU (1 AMU = 1. 66 x 10 -24 gm)

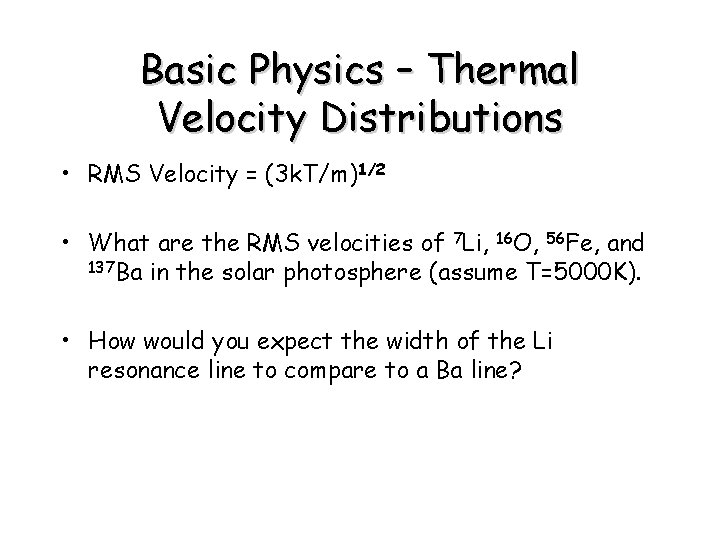
Basic Physics – Thermal Velocity Distributions • RMS Velocity = (3 k. T/m)1/2 • What are the RMS velocities of 7 Li, 16 O, 56 Fe, and 137 Ba in the solar photosphere (assume T=5000 K). • How would you expect the width of the Li resonance line to compare to a Ba line?

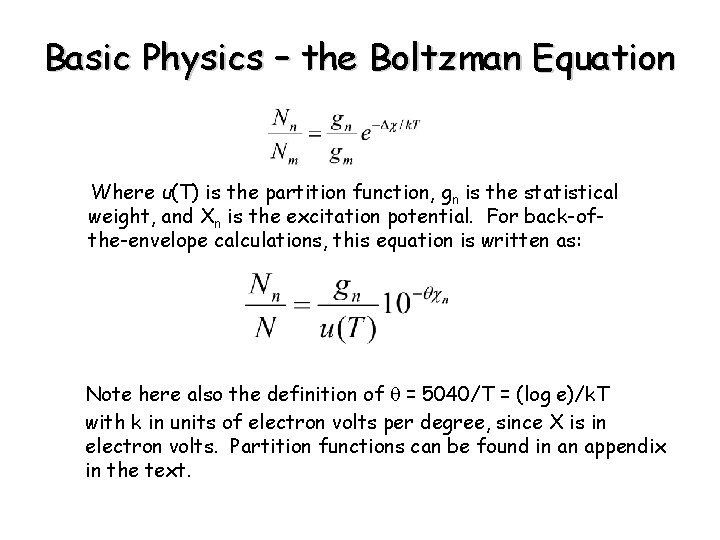
Basic Physics – the Boltzman Equation Where u(T) is the partition function, gn is the statistical weight, and Xn is the excitation potential. For back-ofthe-envelope calculations, this equation is written as: Note here also the definition of q = 5040/T = (log e)/k. T with k in units of electron volts per degree, since X is in electron volts. Partition functions can be found in an appendix in the text.

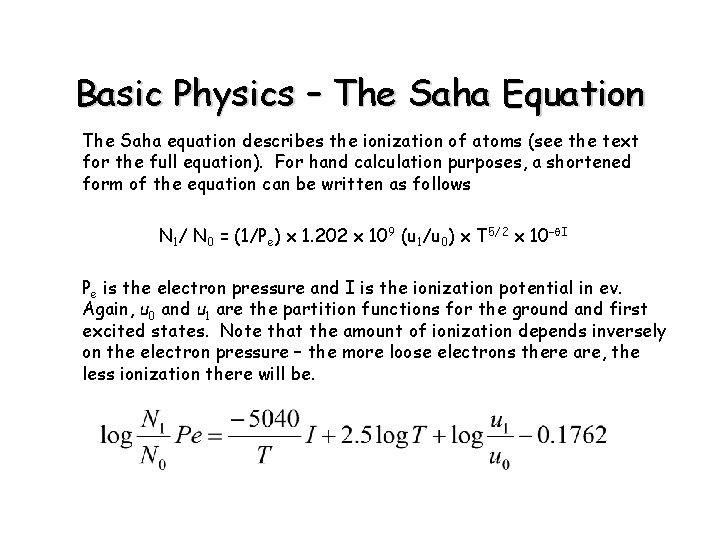
Basic Physics – The Saha Equation The Saha equation describes the ionization of atoms (see the text for the full equation). For hand calculation purposes, a shortened form of the equation can be written as follows N 1/ N 0 = (1/Pe) x 1. 202 x 109 (u 1/u 0) x T 5/2 x 10–q. I Pe is the electron pressure and I is the ionization potential in ev. Again, u 0 and u 1 are the partition functions for the ground and first excited states. Note that the amount of ionization depends inversely on the electron pressure – the more loose electrons there are, the less ionization there will be.

Problems YOU should be able to solve… • Using the ideal gas law, estimate the number density of atoms in the Sun’s photosphere and in the Earth’s atmosphere at sea level. For the Sun, assume T=5000 K, P=105 dyne cm-2. How do the densities compare?

More Problems • During the course of its evolution, the Sun will pass from the main sequence to become a red giant, and then a white dwarf. • Estimate the radius of the Sun in both phases, assuming log g = 1. 0 when the Sun is a red giant, and log g=8 when the Sun is a white dwarf. Assume no mass loss. • Give the answer in both units of the current solar radius and in cgs or MKS units.

More Problems • At (approximately) what Teff is Fe 50% ionized in a main sequence star? In a supergiant? • What is the dominant ionization state of Li in a K giant at 4000 K? In the Sun? In an A star at 8000 K?
 Mat0022
Mat0022 Minor detail example
Minor detail example Minor details examples
Minor details examples Seorang pedagang menjual suatu barang dengan harga 270
Seorang pedagang menjual suatu barang dengan harga 270 Jumlah sudut segilima beraturan
Jumlah sudut segilima beraturan Square root times square root
Square root times square root Norma 540
Norma 540 Crossword puzzle tungkol sa heograpiyang pantao sagot
Crossword puzzle tungkol sa heograpiyang pantao sagot Harga penjualan sebuah tas
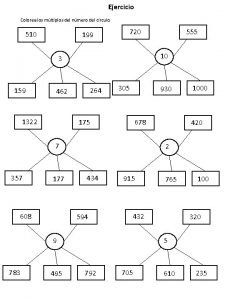
Harga penjualan sebuah tas Colorea los múltiplos del número del círculo
Colorea los múltiplos del número del círculo A 540 degree appraisal involves evaluation by
A 540 degree appraisal involves evaluation by Besar salah satu sudut pusat segi
Besar salah satu sudut pusat segi Cis 540
Cis 540 540 beaumont avenue montréal quebec
540 beaumont avenue montréal quebec What is density in chemistry
What is density in chemistry