25122021 Using Chemistry Fuels 25122021 A fuel is

- Slides: 7

25/12/2021 Using Chemistry

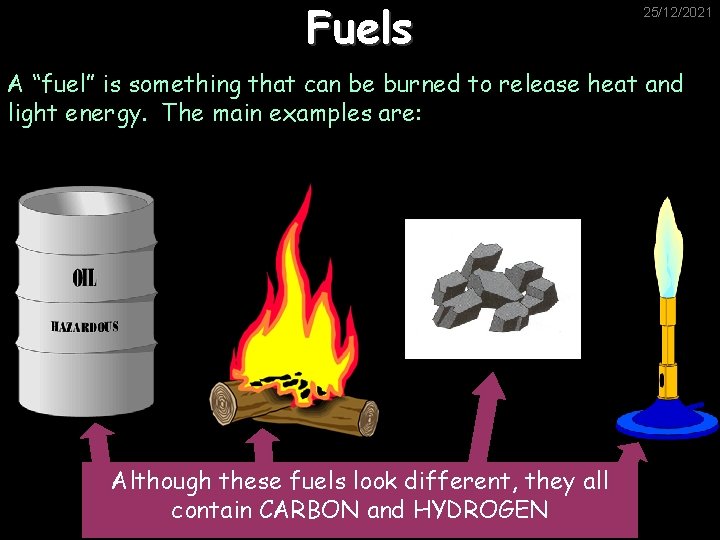
Fuels 25/12/2021 A “fuel” is something that can be burned to release heat and light energy. The main examples are: Although these fuels look different, they all contain CARBON and HYDROGEN

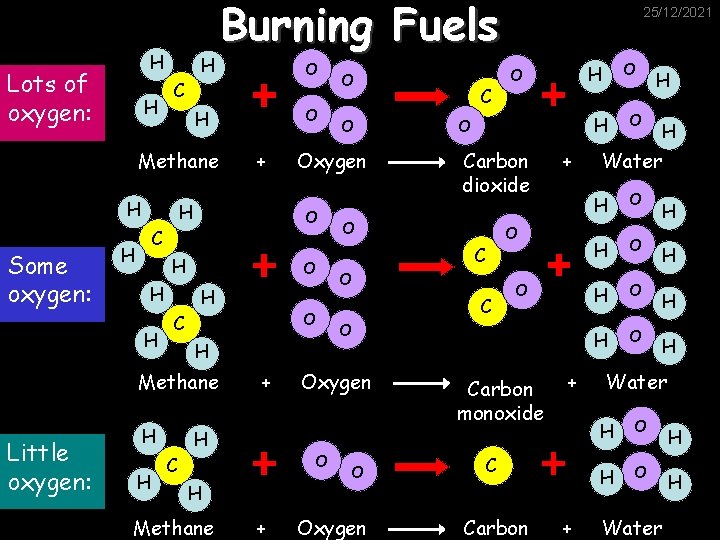
H Lots of oxygen: H H O H H + H H O H H Methane H H O C + H H Methane + O C H Carbon dioxide + O C O Oxygen O H O Oxygen O C H Little oxygen: O C Methane Some oxygen: Burning Fuels 25/12/2021 Carbon monoxide + C Carbon + O H H Water H O H O H H Water H O Water H H

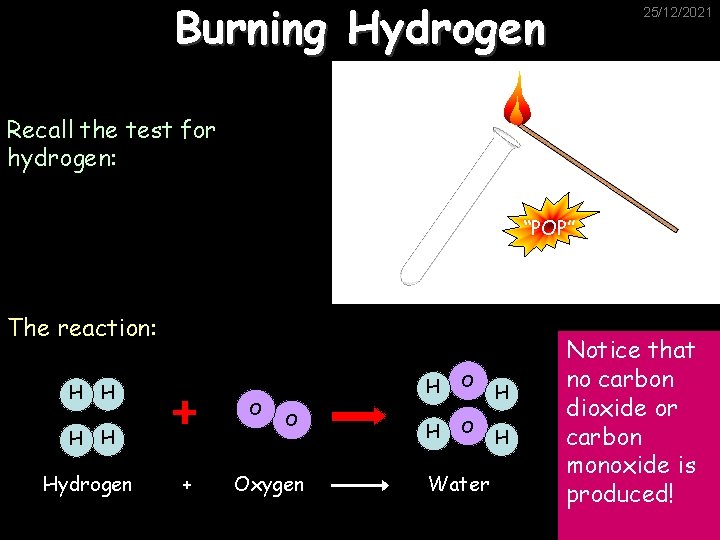
Burning Hydrogen 25/12/2021 Recall the test for hydrogen: “POP” The reaction: H H O H H Hydrogen + O Oxygen H O Water H H Notice that no carbon dioxide or carbon monoxide is produced!

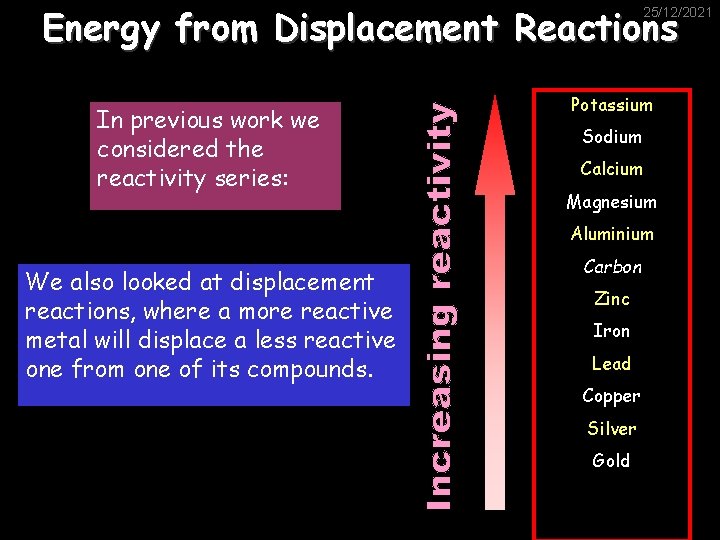
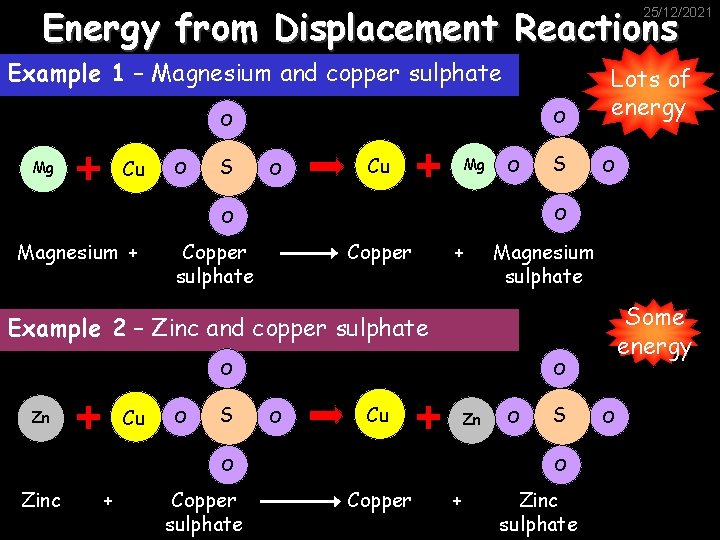
Energy from Displacement Reactions 25/12/2021 In previous work we considered the reactivity series: Potassium Sodium Calcium Magnesium Aluminium We also looked at displacement reactions, where a more reactive metal will displace a less reactive one from one of its compounds. Carbon Zinc Iron Lead Copper Silver Gold

Energy from Displacement Reactions 25/12/2021 Example 1 – Magnesium and copper sulphate O O Cu Mg O S O Cu Mg O O Magnesium + S Lots of energy Copper sulphate Copper + Magnesium sulphate Some energy Example 2 – Zinc and copper sulphate O Cu Zn O S O O Cu Zn O Zinc + Copper sulphate O S O Copper + Zinc sulphate O

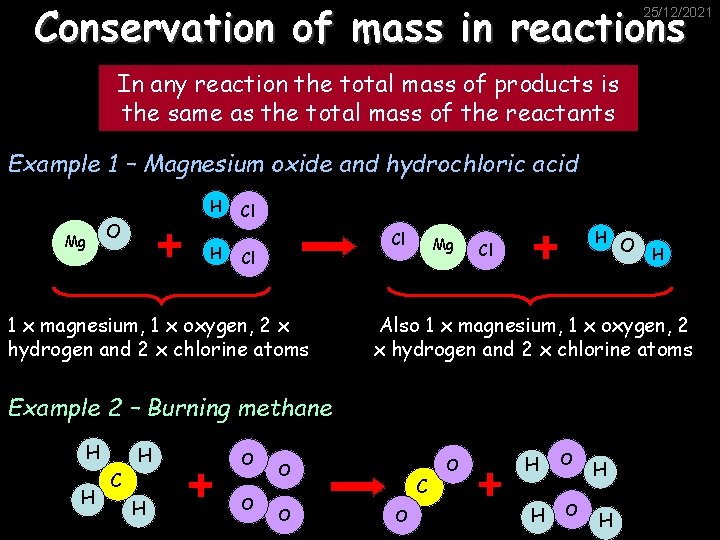
Conservation of mass in reactions 25/12/2021 In any reaction the total mass of products is the same as the total mass of the reactants Example 1 – Magnesium oxide and hydrochloric acid H Mg O H Cl Cl Cl 1 x magnesium, 1 x oxygen, 2 x hydrogen and 2 x chlorine atoms Mg H Cl Also 1 x magnesium, 1 x oxygen, 2 x hydrogen and 2 x chlorine atoms Example 2 – Burning methane H H H C H O O O H C O O H H













