2 Last week 1 Last Lesson What could








- Slides: 17

2. Last week 1. Last Lesson What could cause loss of mass in a chemical reaction? 5 Why can’t a viral infection be treated with antibiotics? 5. Challenge What is an isotope? 3. Last topic What are the symptoms of gonorrhoea? 4. Last Year State the four major blood vessels of the heart.

2. Last week 1. Last Lesson A product which is a What could cause lossgas ofescaping. mass in a chemical reaction? 5 Why can’t a viral Wont work on infection be a virus treated with antibiotics? 5. Challenge 3. Last topic Pain when What are the urinating, symptoms of gonorrhoea? Yellow discharge. A version of an What is an element that has different number isotope? of neutrons. 4. Last Year Vena Cava State the four major Aorta blood vessels of the Pulmonary vein heart. Pulmonary Artery

Concentrations of Solutions and Uncertainty December 2021

Explain how uncertainty of results can be represented. Define the term Concentration. Calculate the mass of a solute in a given volume of solution of known concentration. Calculate range and mean of a set of results as a measurement of uncertainty.

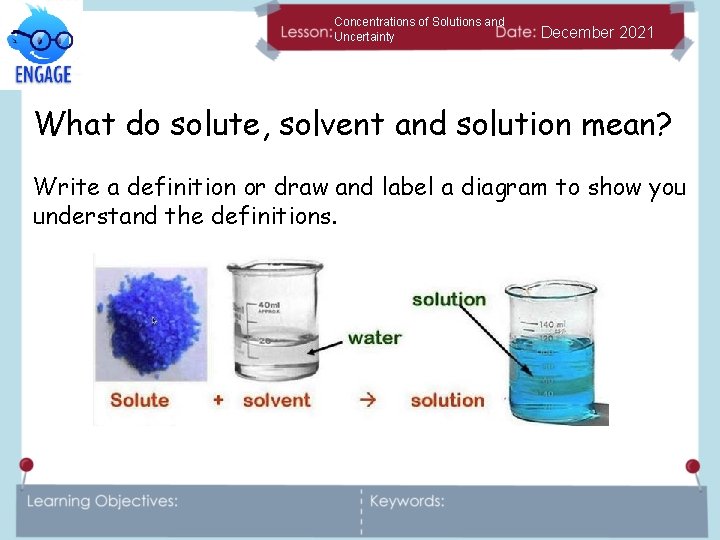
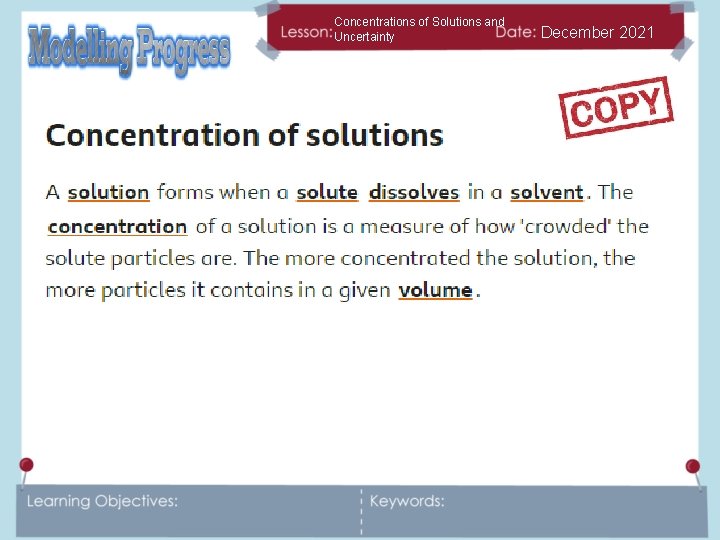
Concentrations of Solutions and Uncertainty December 2021 What do solute, solvent and solution mean? Write a definition or draw and label a diagram to show you understand the definitions.

Concentrations of Solutions and Uncertainty December 2021 What does concentration mean? Can you think of an example of a concentrated and dilute solution?

Concentrations of Solutions and Uncertainty December 2021

Concentrations of Solutions and Uncertainty December 2021 The concentration of a solution can be measured in mass per given volume of solution, eg grams per dm 3 (g/dm 3 ). Concentration can be worked out in g/dm 3 by dividing the mass by volume.

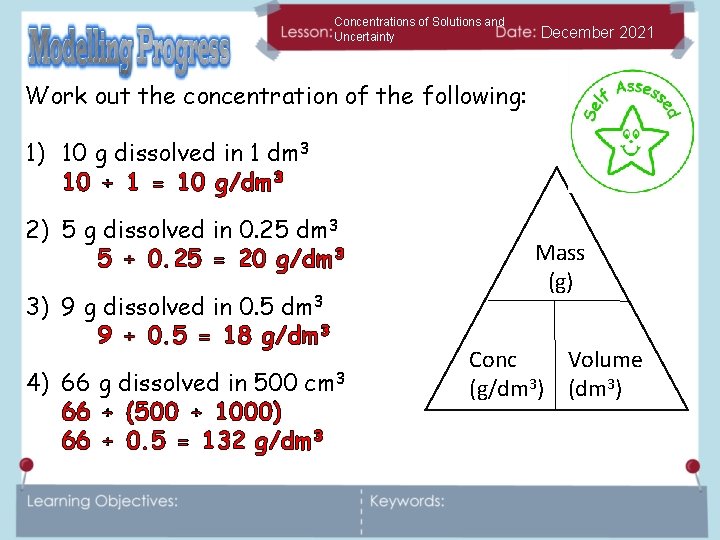
Concentrations of Solutions and Uncertainty December 2021 Work out the concentration of the following: 1) 10 g dissolved in 1 dm 3 10 ÷ 1 = 10 g/dm 3 2) 5 g dissolved in 0. 25 dm 3 5 ÷ 0. 25 = 20 g/dm 3 3) 9 g dissolved in 0. 5 dm 3 9 ÷ 0. 5 = 18 g/dm 3 4) 66 g dissolved in 500 cm 3 66 ÷ (500 ÷ 1000) 66 ÷ 0. 5 = 132 g/dm 3 Mass (g) Conc Volume (g/dm 3) (dm 3)

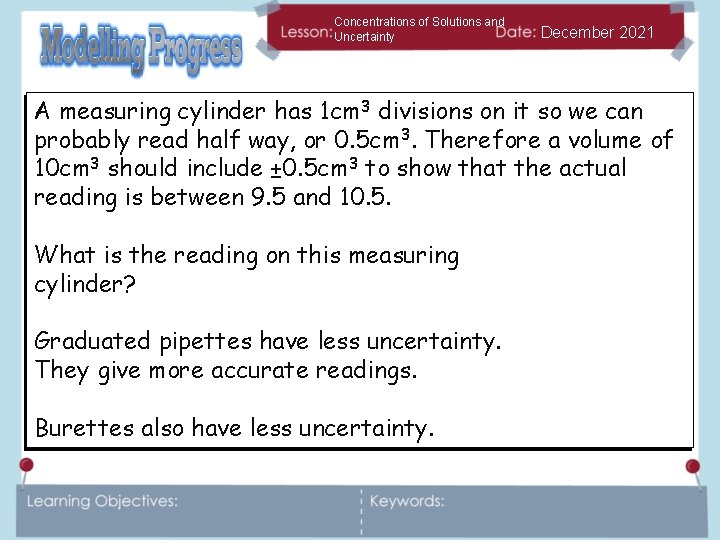
Concentrations of Solutions and Uncertainty December 2021 A measuring cylinder has 1 cm 3 divisions on it so we can probably read half way, or 0. 5 cm 3. Therefore a volume of 10 cm 3 should include ± 0. 5 cm 3 to show that the actual reading is between 9. 5 and 10. 5. What is the reading on this measuring cylinder? Graduated pipettes have less uncertainty. They give more accurate readings. Burettes also have less uncertainty.

Concentrations of Solutions and Uncertainty December 2021 Draw a histogram showing frequency(number of groups) against volume of dilute HCl for these results. Draw one for group A and one for Group B. Volume of dilute HCl (cm 3) Number of groups in this range of results – Group A Number of groups in this range of results – Group B 12. 0 – 12. 9 0 3 13. 0 – 13. 9 3 4 14. 0 – 14. 9 8 3 15. 0 – 15. 9 4 1 16. 0 – 16. 9 0 4 Which group has the highest uncertainty? How do we know?

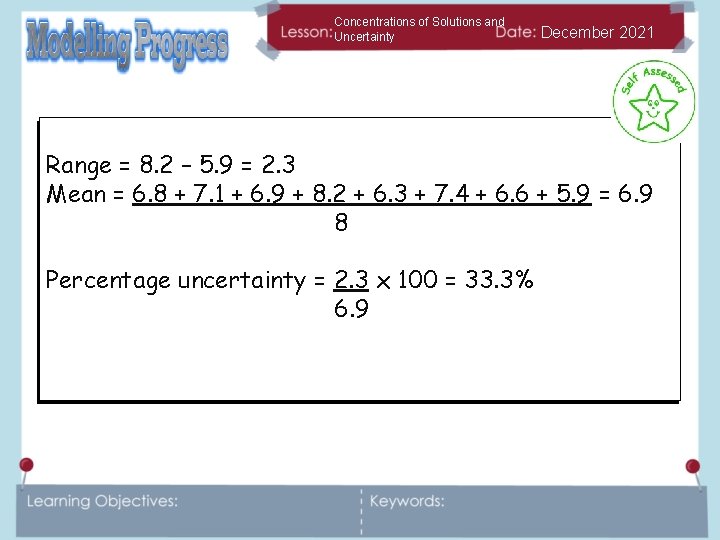
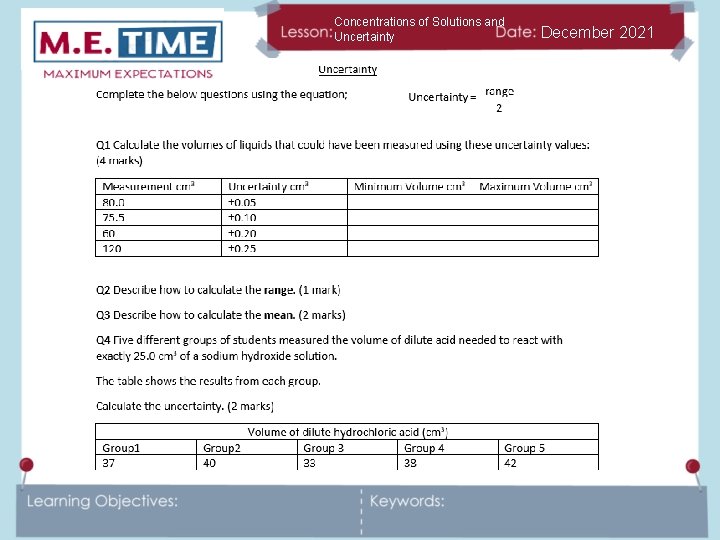
Concentrations of Solutions and Uncertainty December 2021 We can calculate percentage uncertainty according to the following equation: Percentage uncertainty = range of measurements x 100 mean Looking at our results for the mass of the water can you calculate the percentage uncertainty in those results? Calculate the uncertainty in these results. They are all in cm 3 6. 8 7. 1 6. 9 8. 2 6. 3 7. 4 6. 6 5. 9

Concentrations of Solutions and Uncertainty December 2021 Range = 8. 2 – 5. 9 = 2. 3 Mean = 6. 8 + 7. 1 + 6. 9 + 8. 2 + 6. 3 + 7. 4 + 6. 6 + 5. 9 = 6. 9 8 Percentage uncertainty = 2. 3 x 100 = 33. 3% 6. 9

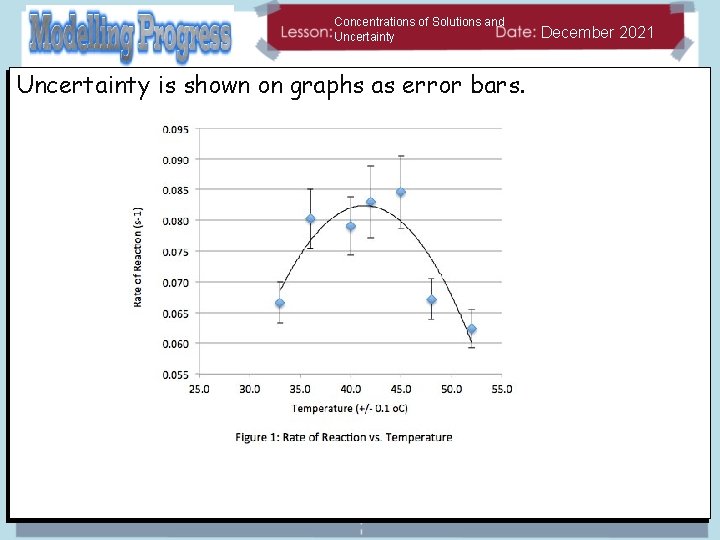
Concentrations of Solutions and Uncertainty is shown on graphs as error bars. December 2021

Concentrations of Solutions and Uncertainty December 2021

Concentrations of Solutions and Uncertainty December 2021 What is meant by the range of results? How do we represent uncertainty in measurements What is the uncertainty in a measuring cylinder that measures in divisions of 1 cm 3? What is the percentage uncertainty for this set of results? 4. 6 g 7 g 5. 5 g 6. 3 g

Concentrations of Solutions and Uncertainty Todays Lesson Last Lesson December 2021 What is concentration? How is concentration calculated? Why might the Law of conservation of mass not be observed in a chemical reaction within a non-closed system? Which number on the Periodic table is the relative atomic mass number?