2 Last week 1 Last Lesson What is





- Slides: 15

2. Last week 1. Last Lesson What is the law of conservation of mass? 5 Why do diamond and graphite have a high MP? 5. Challenge What is an isotope? 3. Last topic Why do veins have valves? 4. Last Year How did Mendeleev overcome the problems he encountered to produce his periodic table?

2. Last week 1. Last Lesson Amount of of What is the law reactants =of conservation amount of mass? products 5 Why do Many diamond and covalent graphite have bonds a high MP? 5. Challenge 3. Last topic To prevent back Why do veins have flow due to the low valves? pressure. A version of an What is an element that has different number isotope? of neutrons. 4. Last Year By leaving gaps for How did Mendeleev elements that heproblems thought overcome the had not been discovered he encountered to and in places changed the produce hisonperiodic order based atomic table? weights.

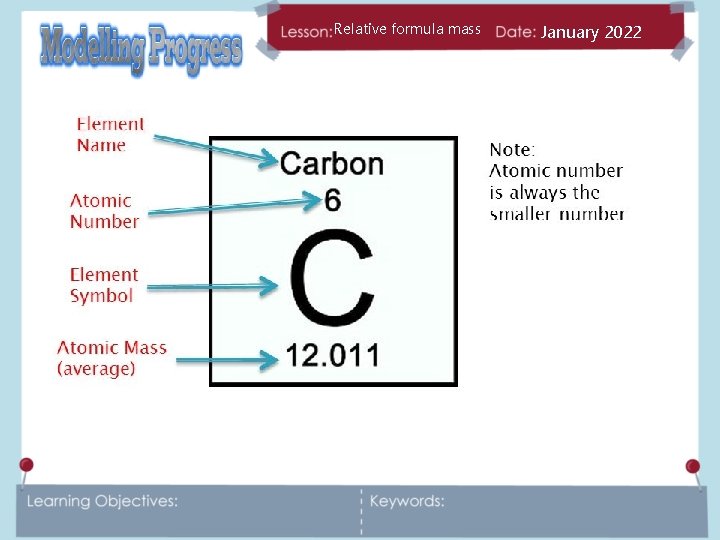
Relative formula mass January 2022

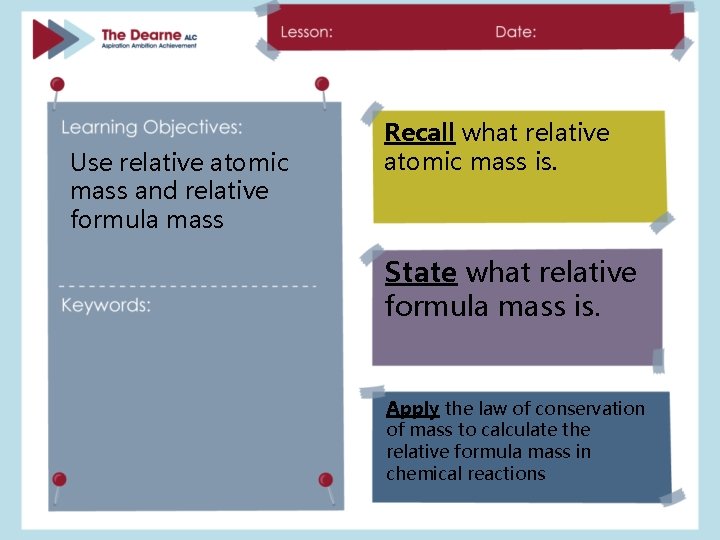
Use relative atomic mass and relative formula mass Recall what relative atomic mass is. State what relative formula mass is. Apply the law of conservation of mass to calculate the relative formula mass in chemical reactions

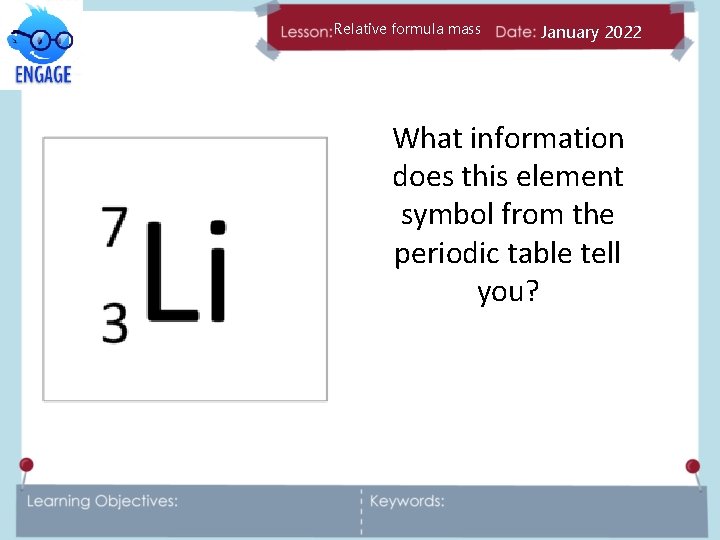
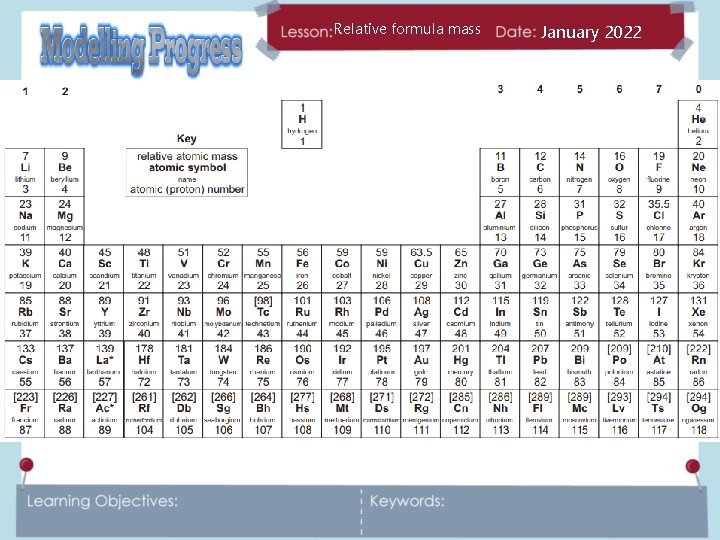
Relative formula mass January 2022 What information does this element symbol from the periodic table tell you?

Relative formula mass January 2022

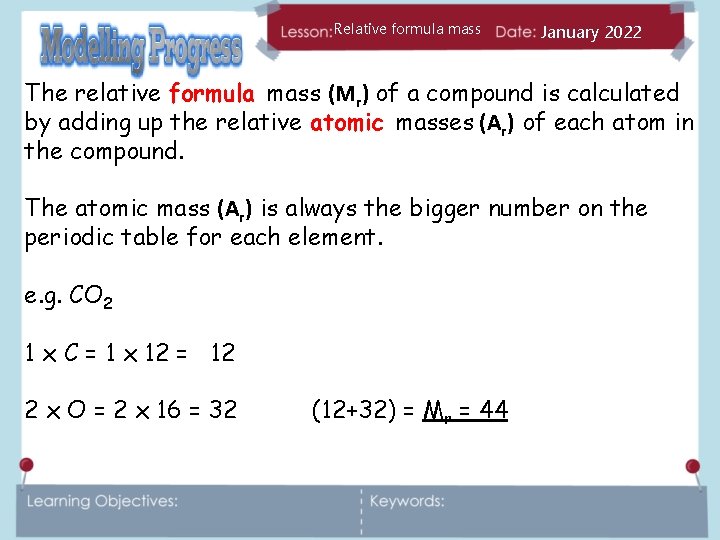
Relative formula mass January 2022 The relative formula mass (Mr) of a compound is calculated by adding up the relative atomic masses (Ar) of each atom in the compound. The atomic mass (Ar) is always the bigger number on the periodic table for each element. e. g. CO 2 1 x C = 1 x 12 = 12 2 x O = 2 x 16 = 32 (12+32) = Mr = 44

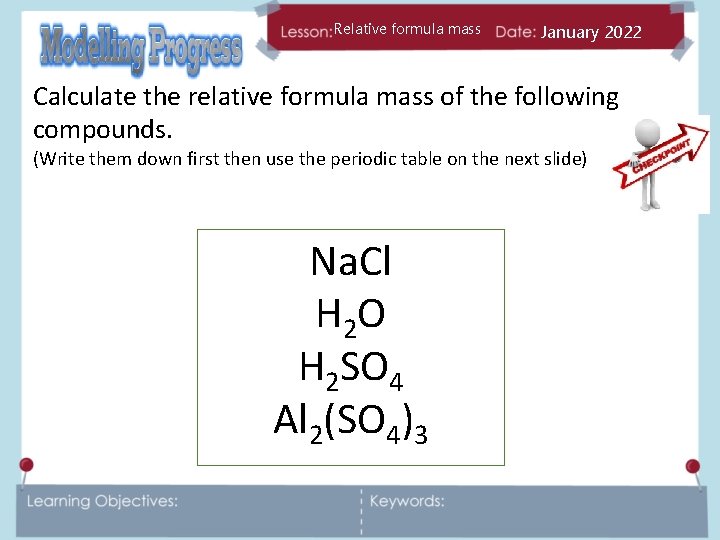
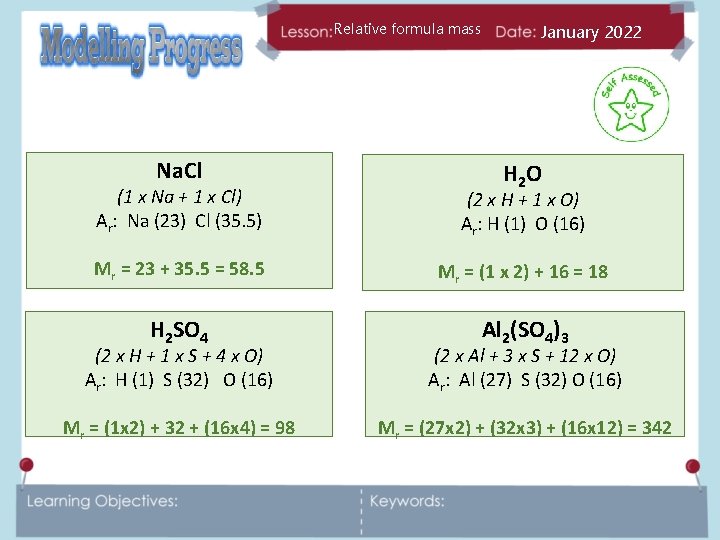
Relative formula mass January 2022 Calculate the relative formula mass of the following compounds. (Write them down first then use the periodic table on the next slide) Na. Cl H 2 O H 2 SO 4 Al 2(SO 4)3

Relative formula mass January 2022

Relative formula mass Na. Cl January 2022 H 2 O (1 x Na + 1 x Cl) Ar: Na (23) Cl (35. 5) (2 x H + 1 x O) Ar: H (1) O (16) Mr = 23 + 35. 5 = 58. 5 Mr = (1 x 2) + 16 = 18 H 2 SO 4 Al 2(SO 4)3 (2 x H + 1 x S + 4 x O) Ar: H (1) S (32) O (16) (2 x Al + 3 x S + 12 x O) Ar: Al (27) S (32) O (16) Mr = (1 x 2) + 32 + (16 x 4) = 98 Mr = (27 x 2) + (32 x 3) + (16 x 12) = 342

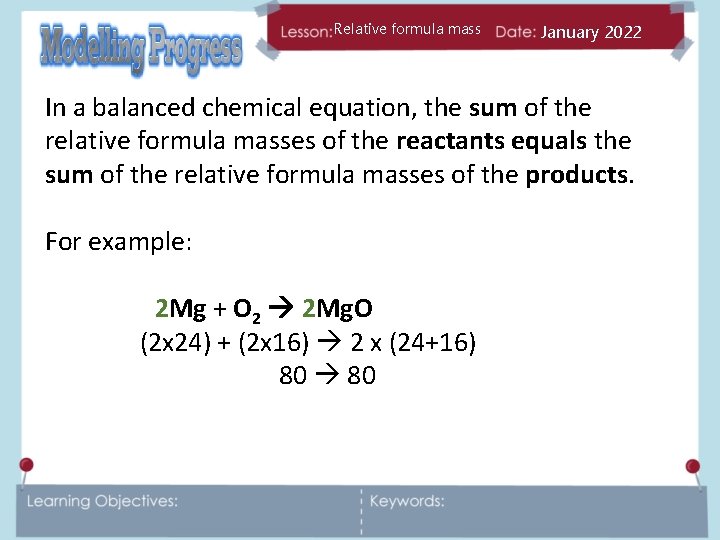
Relative formula mass January 2022 In a balanced chemical equation, the sum of the relative formula masses of the reactants equals the sum of the relative formula masses of the products. For example: 2 Mg + O 2 2 Mg. O (2 x 24) + (2 x 16) 2 x (24+16) 80

Relative formula mass January 2022

Relative formula mass January 2022

Relative formula mass January 2022

Relative formula mass Todays Lesson Last Lesson January 2022 Which number on the Periodic table is the relative atomic mass number? How do you calculate the relative formula mass of a compound? Balance the following equation: CO 2 + H 2 O C 6 H 12 O 6 + O 2 Which pathogens cause: Gonorrhoea, HIV, Measles, TMV, Malaria