2 Last week 1 Last Lesson How do








- Slides: 15

2. Last week 1. Last Lesson How do you calculate the relative formula mass? 5 What is meant by communicable disease. Give and example 5. Challenge What is an isotope? 3. Last topic State an adaptation for each blood vessel. 4. Last Year How did Mendeleev overcome the problems he encountered to produce his periodic table?

2. Last week 1. Last Lesson Add do up all of the How you relative atomic calculate the masses for each of relative formula the atoms in the mass? compound 5 Can Whatbe is meant by communicable transmitted disease. Give and from person to example person 5. Challenge 3. Last topic Capillaries – 1 cell State an adaptation thick. Veins – Valves. for each blood vessel. Arteries – small lumen. A version of an What is an element that has different number isotope? of neutrons. 4. Last Year By leaving gaps for How did Mendeleev elements that heproblems thought overcome the had not been discovered he encountered to and in places changed the produce hisonperiodic order based atomic table? weights.

Mass Changes When a Reactant or a Product is a Gas January 2022

Explain any observed changes in mass in nonenclosed systems during a chemical reaction Recall how to balance chemical equations. Identify changes in mass in a nonenclosed system. Apply the particle model to explain changes in mass in non-enclosed systems.

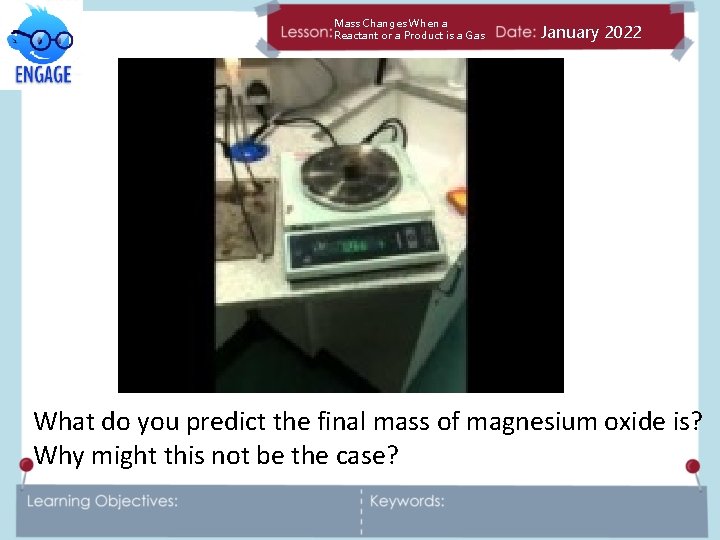
Mass Changes When a Reactant or a Product is a Gas January 2022 What do you predict the final mass of magnesium oxide is? Why might this not be the case?

Mass Changes When a Reactant or a Product is a Gas January 2022 A small amount of marble chips was added to hydrochloric acid in a flask. 25 g of carbon dioxide was produced. The flask and contents originally weighed 270 g. What will be the mass of the flask and its contents now? How do you know?

Mass Changes When a Reactant or a Product is a Gas January 2022 A small amount of marble chips was added to hydrochloric acid in a flask. 25 g of carbon dioxide was produced. The flask and contents originally weighed 270 g. Since the total mass of products (and flask) must be 270 g, and 25 g of this is the carbon dioxide, there must be 270 – 25 = 245 g of products (and flask) left

Mass Changes When a Reactant or a Product is a Gas January 2022

Mass Changes When a Reactant or a Product is a Gas January 2022 Why might mass be lost in a chemical reaction? How could you measure the loss of mass in a chemical reaction? Using the particle model theory explain how mass is lost in a chemical reaction, even though the Law of conservation of mass means this should not be the case.

Mass Changes When a Reactant or a Product is a Gas January 2022

Mass Changes When a Reactant or a Product is a Gas January 2022

Mass Changes When a Reactant or a Product is a Gas January 2022

Mass Changes When a Reactant or a Product is a Gas January 2022

Mass Changes When a Reactant or a Product is a Gas January 2022

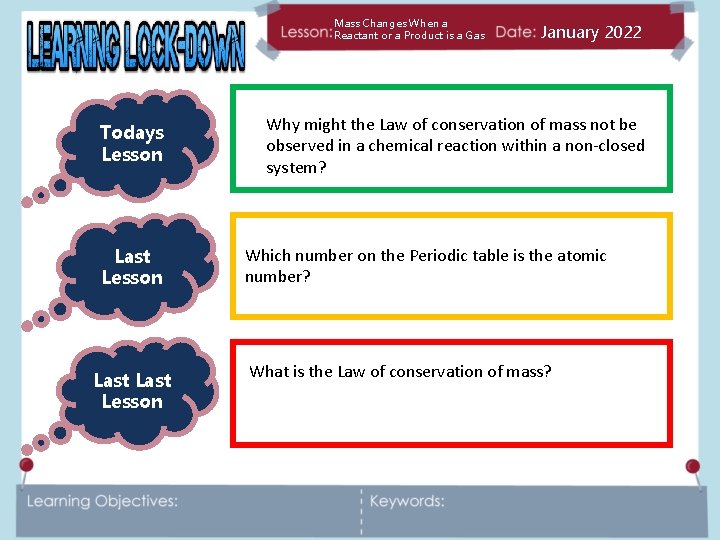
Mass Changes When a Reactant or a Product is a Gas Todays Lesson Last Lesson January 2022 Why might the Law of conservation of mass not be observed in a chemical reaction within a non-closed system? Which number on the Periodic table is the atomic number? What is the Law of conservation of mass?