What makes Mc Donalds fries taste SOOOO good




- Slides: 11

What makes Mc. Donald’s fries taste SOOOO good?

Lipids • • • Lipids are composed of C, H, and O Greater H: O ratio than 2: 1 Primary function is energy storage. Energy is stored in C-H bonds. More efficient in storing energy than carbohydrates • Other functions: waterproofing, membranes in cells, hormones • Macromolecules: Fats, Waxes and Oils.

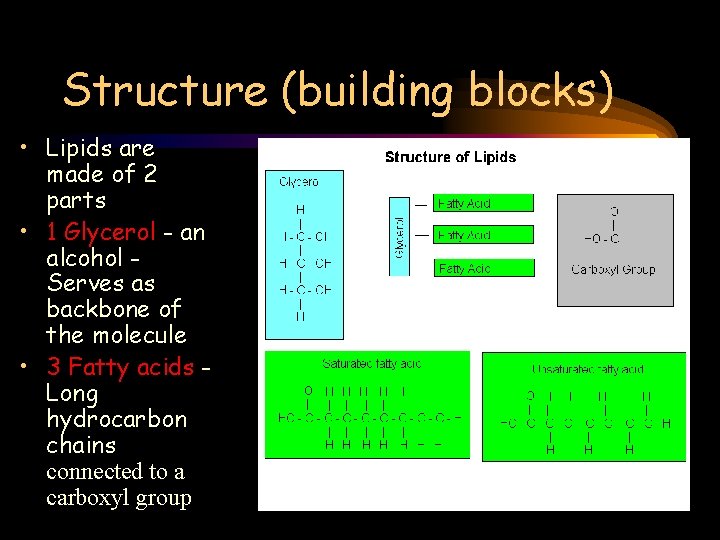
Structure (building blocks) • Lipids are made of 2 parts • 1 Glycerol - an alcohol Serves as backbone of the molecule • 3 Fatty acids Long hydrocarbon chains connected to a carboxyl group


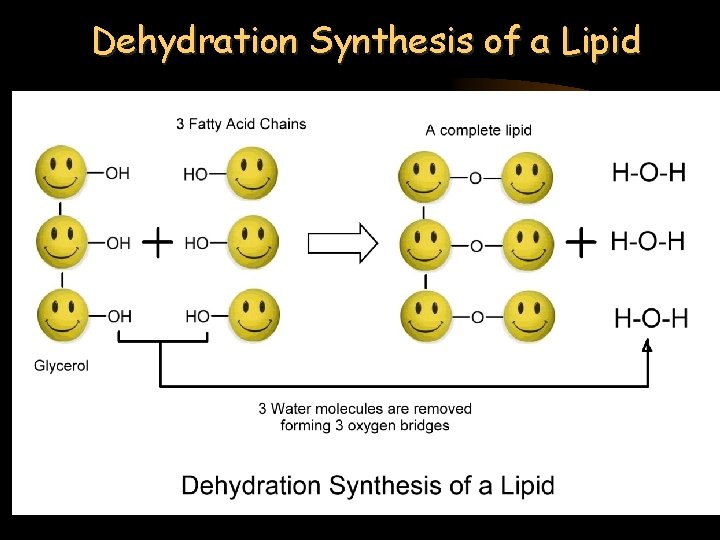
Dehydration Synthesis of a Lipid

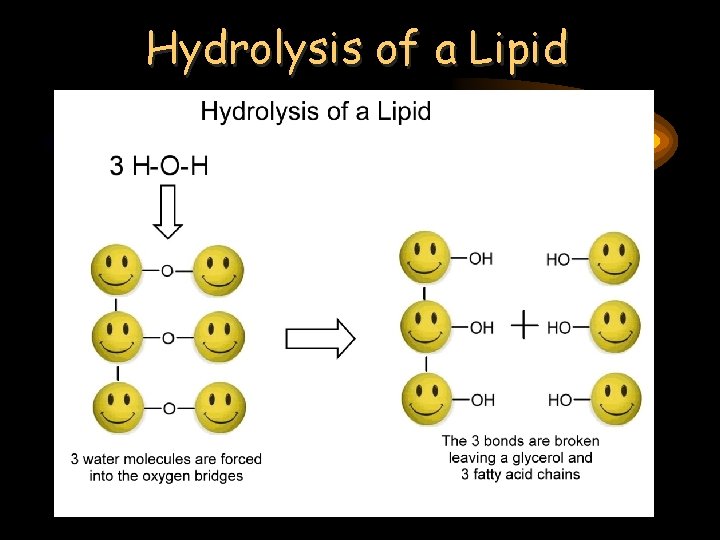
Hydrolysis of a Lipid

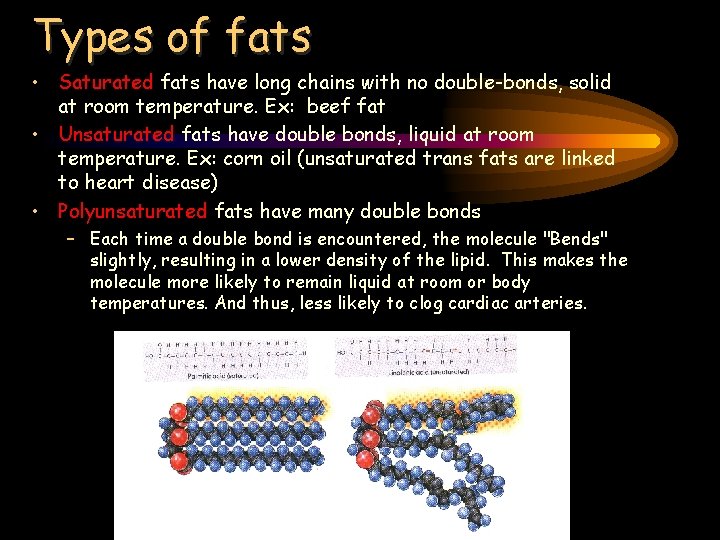
Types of fats • Saturated fats have long chains with no double-bonds, solid at room temperature. Ex: beef fat • Unsaturated fats have double bonds, liquid at room temperature. Ex: corn oil (unsaturated trans fats are linked to heart disease) • Polyunsaturated fats have many double bonds – Each time a double bond is encountered, the molecule "Bends" slightly, resulting in a lower density of the lipid. This makes the molecule more likely to remain liquid at room or body temperatures. And thus, less likely to clog cardiac arteries.

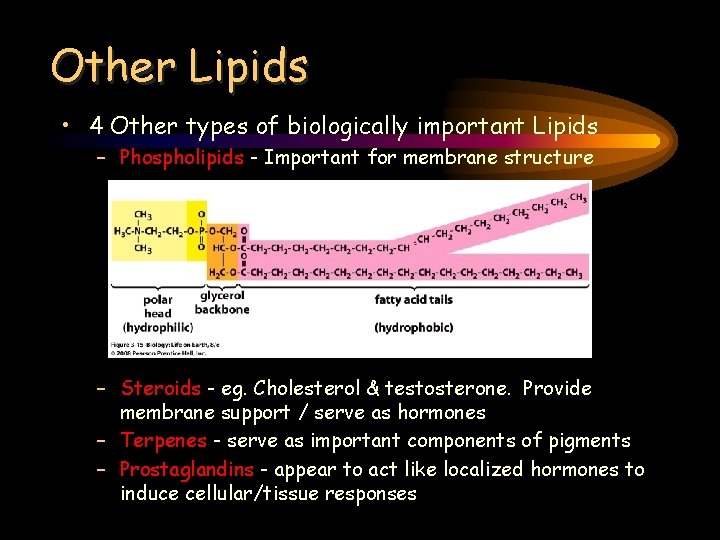
Other Lipids • 4 Other types of biologically important Lipids – Phospholipids - Important for membrane structure – Steroids - eg. Cholesterol & testosterone. Provide membrane support / serve as hormones – Terpenes - serve as important components of pigments – Prostaglandins - appear to act like localized hormones to induce cellular/tissue responses

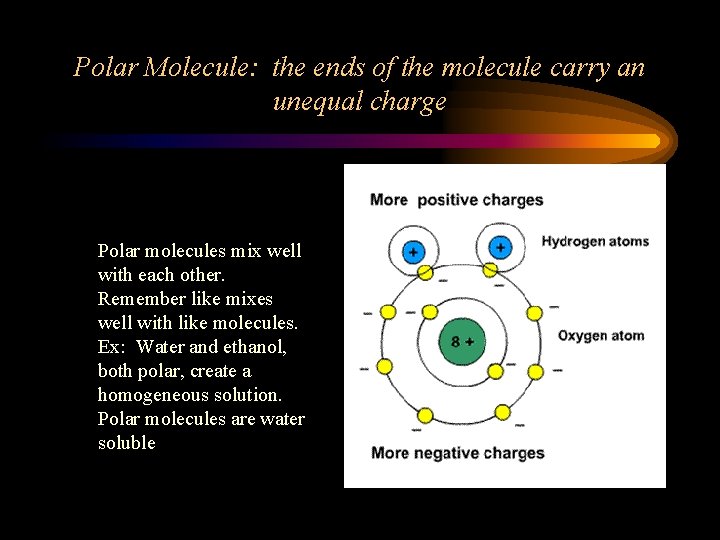
Polar Molecule: the ends of the molecule carry an unequal charge Polar molecules mix well with each other. Remember like mixes well with like molecules. Ex: Water and ethanol, both polar, create a homogeneous solution. Polar molecules are water soluble

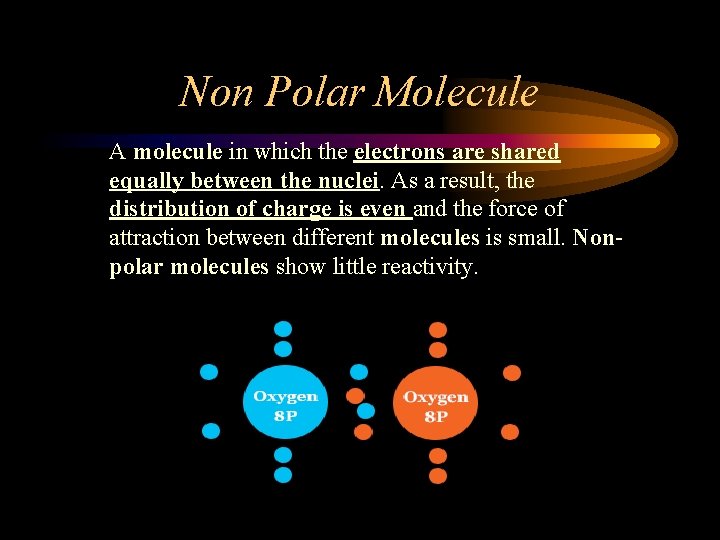
Non Polar Molecule A molecule in which the electrons are shared equally between the nuclei. As a result, the distribution of charge is even and the force of attraction between different molecules is small. Nonpolar molecules show little reactivity.

Let’s Build!
 A fact statement
A fact statement Every good boy deserves fries
Every good boy deserves fries O taste and see that the lord is good chords
O taste and see that the lord is good chords Taste and see that the lord is good
Taste and see that the lord is good Taste and see that the lord is good
Taste and see that the lord is good Oh taste and see the lord is good
Oh taste and see the lord is good You are good you are good when theres nothing good in me
You are good you are good when theres nothing good in me Good thoughts good words good deeds meaning
Good thoughts good words good deeds meaning Cómo se dice buenas tardes
Cómo se dice buenas tardes Hello teacher good afternoon
Hello teacher good afternoon Good afternoon buenas tardes
Good afternoon buenas tardes Slogan i'm lovin it
Slogan i'm lovin it





















