Thermodynamics II Engines and Refrigerators HEAT ENGINE REFRIGERATOR


- Slides: 8

Thermodynamics II

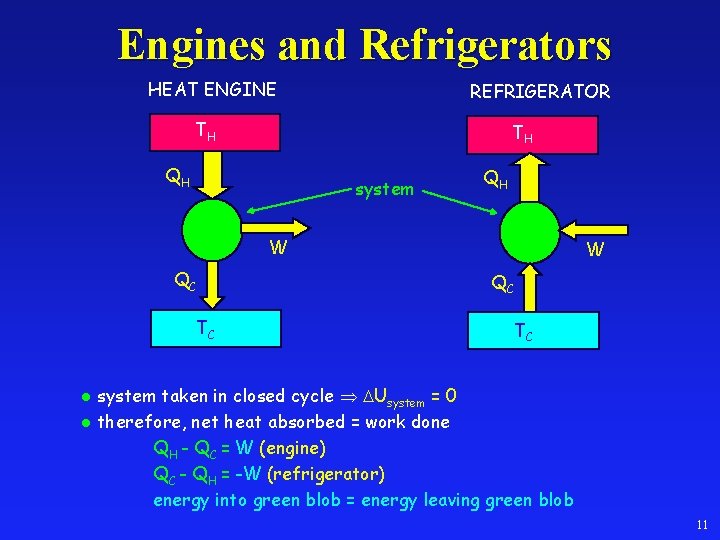
Engines and Refrigerators HEAT ENGINE REFRIGERATOR TH TH QH system QH W QC TC system taken in closed cycle Usystem = 0 l therefore, net heat absorbed = work done QH - QC = W (engine) QC - QH = -W (refrigerator) energy into green blob = energy leaving green blob l 11

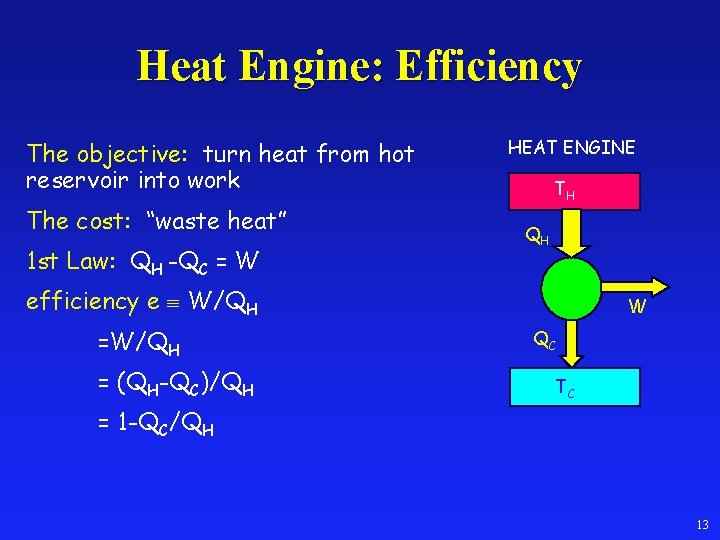
Heat Engine: Efficiency The objective: turn heat from hot reservoir into work The cost: “waste heat” 1 st Law: QH -QC = W HEAT ENGINE TH QH efficiency e W/QH = (QH-QC)/QH W QC TC = 1 -QC/QH 13

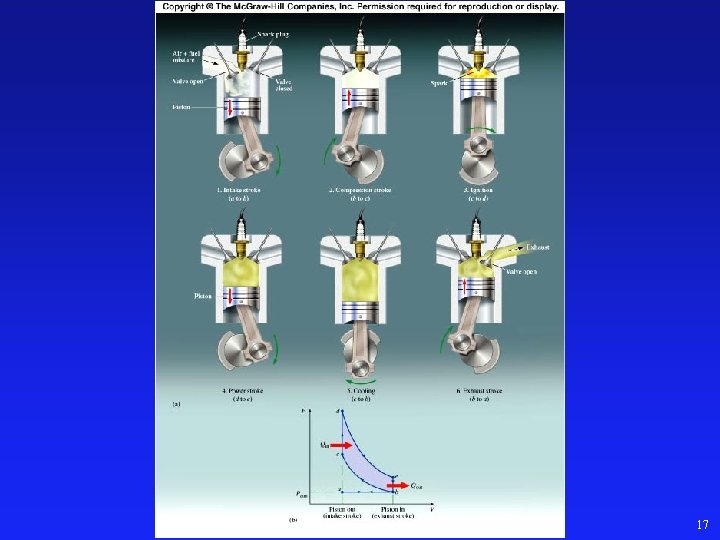
17

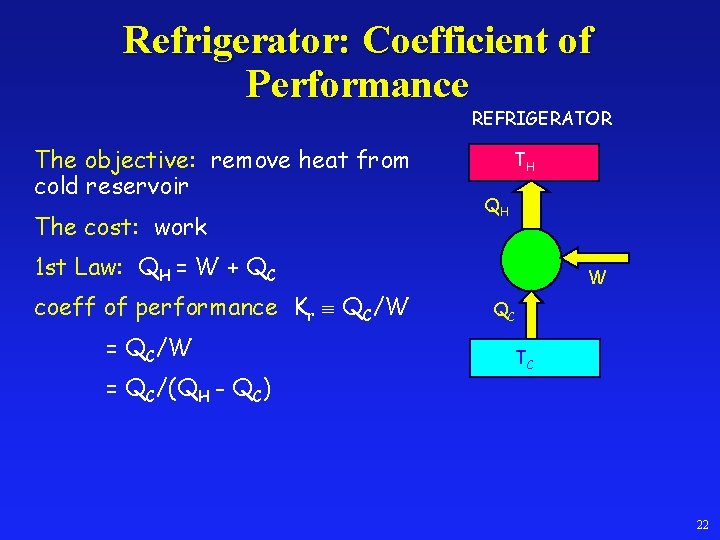
Refrigerator: Coefficient of Performance REFRIGERATOR The objective: remove heat from cold reservoir The cost: work TH QH 1 st Law: QH = W + QC coeff of performance Kr QC/W = QC/(QH - QC) W QC TC 22

Carnot Cycle l Idealized Heat Engine èNo Friction è S = Q/T = 0 èReversible Process » Isothermal Expansion » Adiabatic Expansion » Isothermal Compression » Adiabatic Compression 32

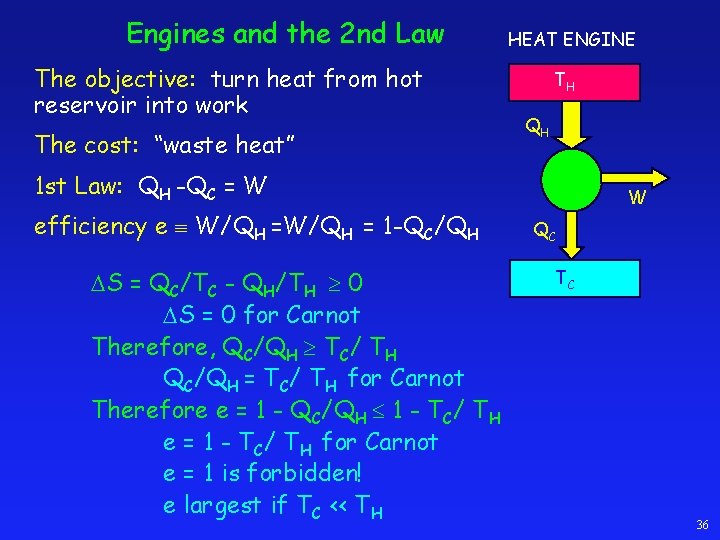
Engines and the 2 nd Law The objective: turn heat from hot reservoir into work The cost: “waste heat” HEAT ENGINE TH QH 1 st Law: QH -QC = W efficiency e W/QH = 1 -QC/QH S = QC/TC - QH/TH 0 S = 0 for Carnot Therefore, QC/QH TC/ TH QC/QH = TC/ TH for Carnot Therefore e = 1 - QC/QH 1 - TC/ TH e = 1 - TC/ TH for Carnot e = 1 is forbidden! e largest if TC << TH W QC TC 36

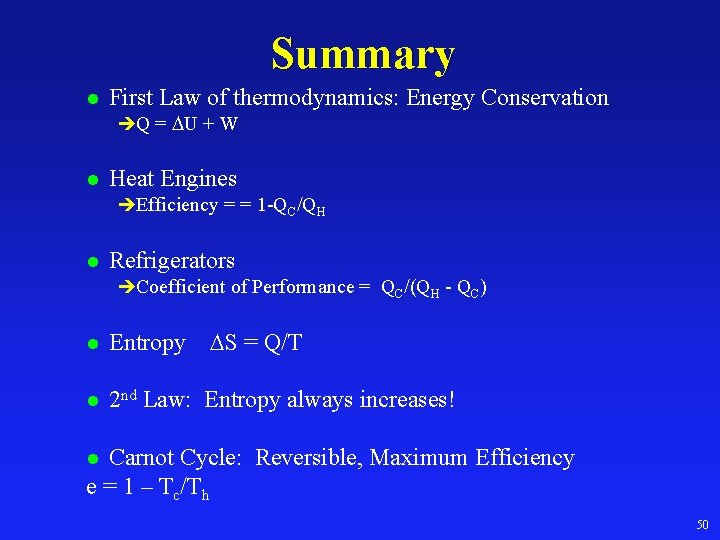
Summary l First Law of thermodynamics: Energy Conservation èQ = U + W l Heat Engines èEfficiency = = 1 -QC/QH l Refrigerators èCoefficient of Performance = QC/(QH - QC) S = Q/T l Entropy l 2 nd Law: Entropy always increases! Carnot Cycle: Reversible, Maximum Efficiency e = 1 – Tc/Th l 50
 Difference between heat engine and refrigerator
Difference between heat engine and refrigerator Refrigeration definition thermodynamics
Refrigeration definition thermodynamics Refrigeration
Refrigeration Refrigeration t-s diagram
Refrigeration t-s diagram Open air refrigeration system
Open air refrigeration system Brick refrigerators
Brick refrigerators External and internal combustion engine
External and internal combustion engine Your refrigerator and pantry are the major items here.
Your refrigerator and pantry are the major items here. Chapter 5 principles of engine operation
Chapter 5 principles of engine operation