Thermodynamic Models of Magmas Lecture 13 Silicate Magmas



- Slides: 8

Thermodynamic Models of Magmas Lecture 13

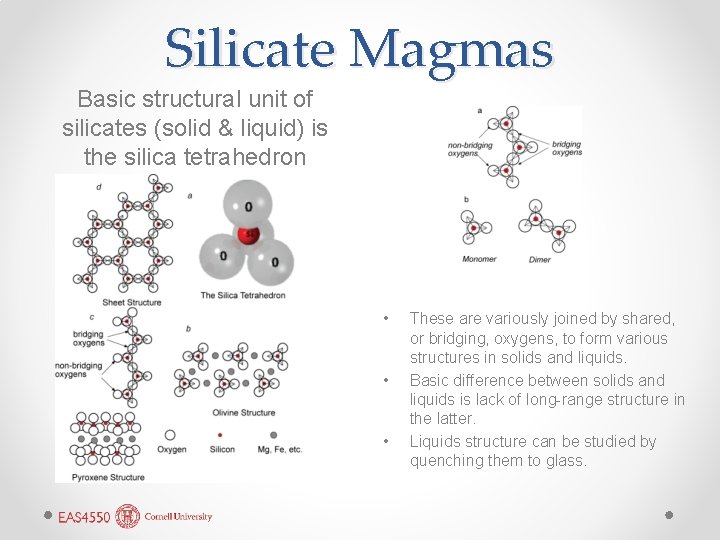
Silicate Magmas Basic structural unit of silicates (solid & liquid) is the silica tetrahedron • • • These are variously joined by shared, or bridging, oxygens, to form various structures in solids and liquids. Basic difference between solids and liquids is lack of long-range structure in the latter. Liquids structure can be studied by quenching them to glass.

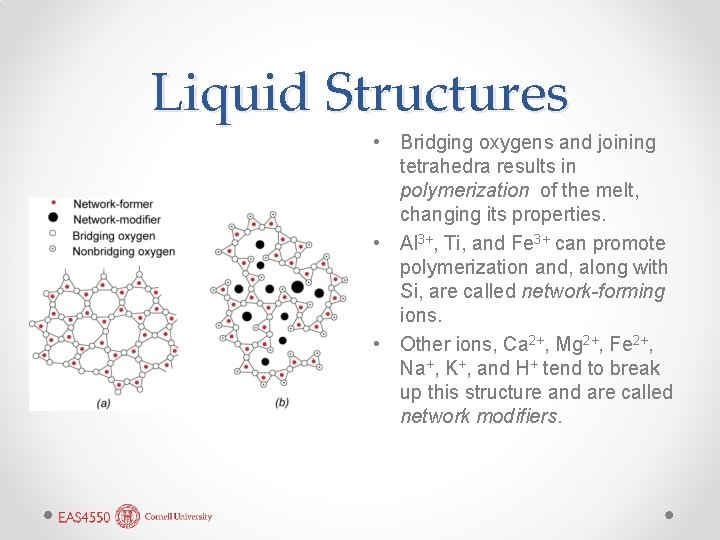
Liquid Structures • Bridging oxygens and joining tetrahedra results in polymerization of the melt, changing its properties. • Al 3+, Ti, and Fe 3+ can promote polymerization and, along with Si, are called network-forming ions. • Other ions, Ca 2+, Mg 2+, Fe 2+, Na+, K+, and H+ tend to break up this structure and are called network modifiers.

Modeling Silicate Liquids • Silicate liquids are complex solutions of many components. • Solids crystallizing from them are generally solutions themselves. • Generally these solutions cannot be treated as ideal. • Crystallization (or melting) occurs over a wide range of T (400 -500˚C). • Problems are: o o • • Decide on the components Determine the nature of the model • Ghiorso et al. adopt a regular solution model for their MELTS model. Determine the interaction parameters from experimental data. The resulting program then iteratively computes free energy of the liquid plus free energy of all possible precipitating solids and calculates the equilibrium assemblage based on the principles that o o the stable assemblage is the one with the lowest free energy. The chemical potentials of components in coexisting phases are equal.

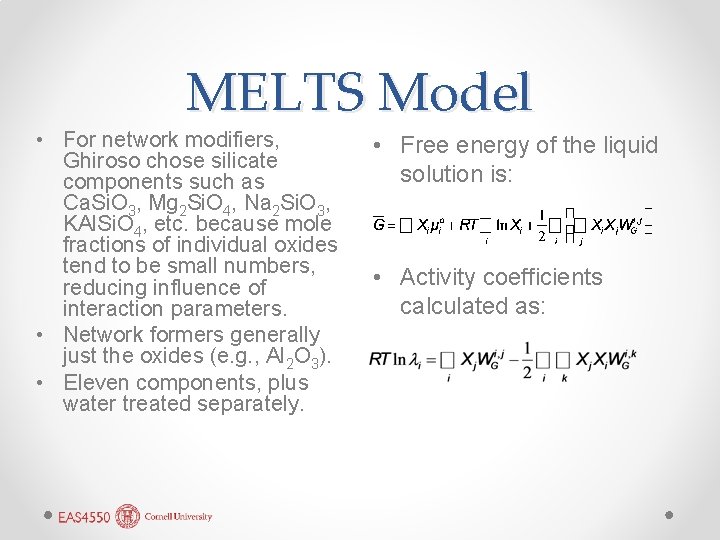
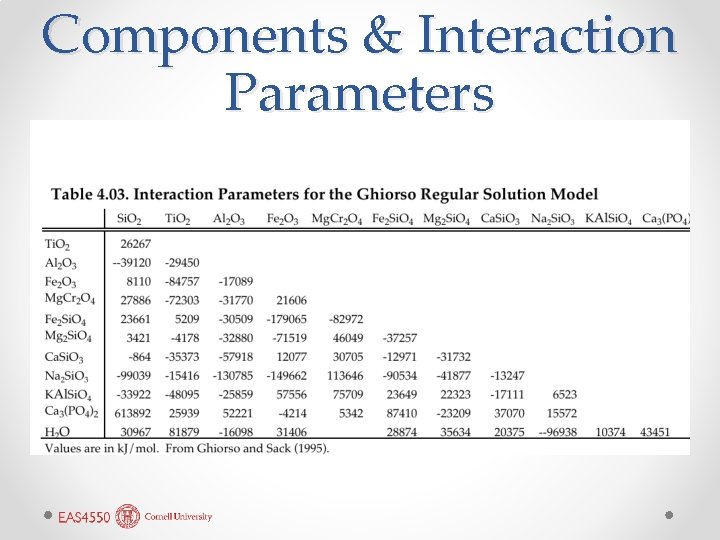
MELTS Model • For network modifiers, Ghiroso chose silicate components such as Ca. Si. O 3, Mg 2 Si. O 4, Na 2 Si. O 3, KAl. Si. O 4, etc. because mole fractions of individual oxides tend to be small numbers, reducing influence of interaction parameters. • Network formers generally just the oxides (e. g. , Al 2 O 3). • Eleven components, plus water treated separately. • Free energy of the liquid solution is: • Activity coefficients calculated as:

Components & Interaction Parameters

Will Plagioclase Precipitate? • For anorthite, reaction of interest is: Ca. Si. O 3(l) + Al 2 O 3(l) + Si. O 2(l) ⇋ Ca. Al 2 Si 2 O 8(plag) • But plag is usually a solid solution, so: • x[½Na. Si. O 3 + ½Al 2 O 3(l) + 2½Si. O 2(l)] + y[Ca. Si. O 3(l) + Al 2 O 3(l) + Si. O 2(l)] ⇋ [y. Ca. Al 2 Si 2 O 8–x. Na. Al. Si 3 O 8](plag) • What thermodynamic condition must be met for plagioclase to precipitate? o ∆Gr is negative. o The negative of ∆Gr is often referred to (particularly in MELTS lingo) as the affinity of reaction, Ar. o Melts calculates affinities for all possible reactions. • If a plagioclase crystal has precipitated from a magma under equilibrium conditions, what can we say about the component? o Chemical potentials of components must be equal in both solid and liquid.

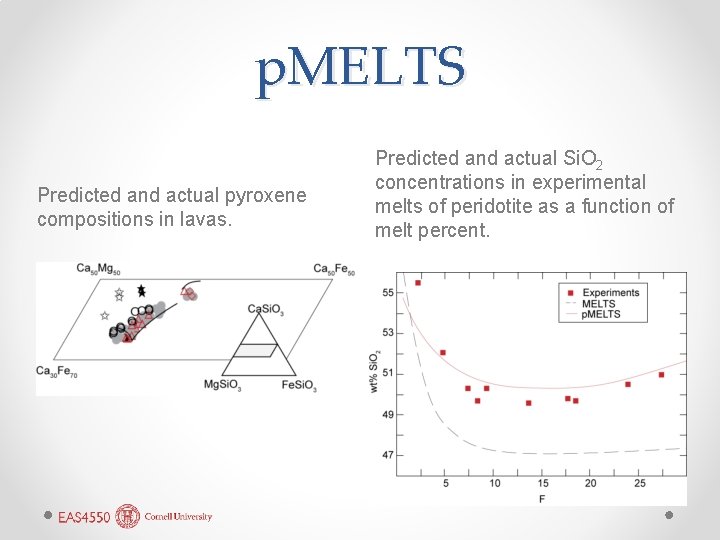
p. MELTS Predicted and actual pyroxene compositions in lavas. Predicted and actual Si. O 2 concentrations in experimental melts of peridotite as a function of melt percent.