Synthesis of Nanomaterials SolGel Hydrothermal Solvothermal Solgel method











- Slides: 18

Synthesis of Nanomaterials – Sol-Gel Hydrothermal Solvothermal

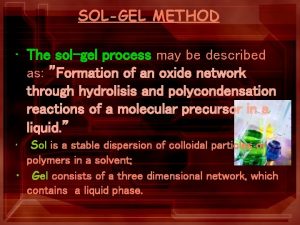
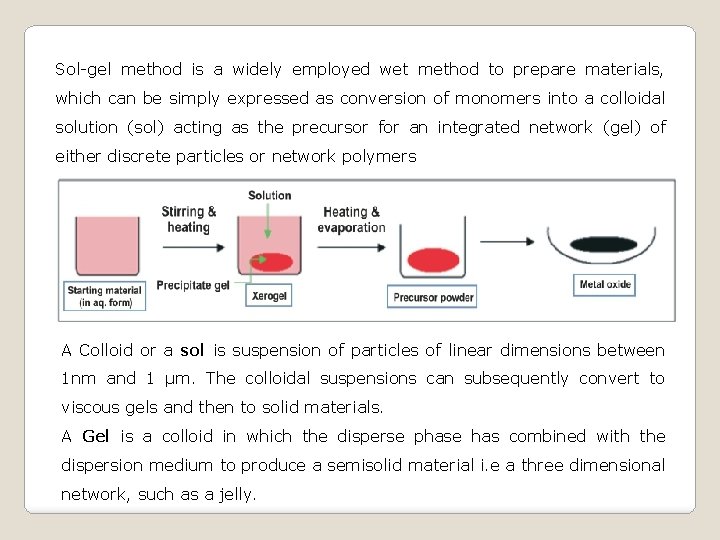
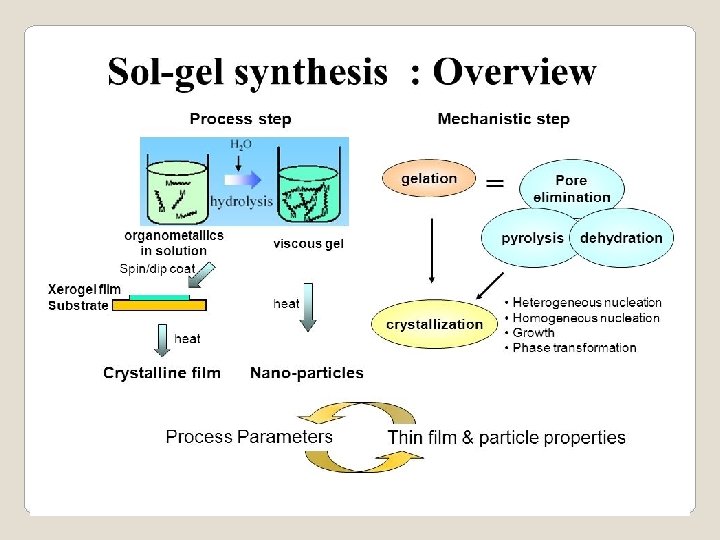
Sol-gel method is a widely employed wet method to prepare materials, which can be simply expressed as conversion of monomers into a colloidal solution (sol) acting as the precursor for an integrated network (gel) of either discrete particles or network polymers A Colloid or a sol is suspension of particles of linear dimensions between 1 nm and 1 μm. The colloidal suspensions can subsequently convert to viscous gels and then to solid materials. A Gel is a colloid in which the disperse phase has combined with the dispersion medium to produce a semisolid material i. e a three dimensional network, such as a jelly.

The sol-gel process, also known as chemical solution deposition, is a wet-chemical technique widely used in the fields of materials science and ceramic engineering. Such methods are used primarily for the fabrication of materials (typically a metal oxide) starting from a chemical solution (or sol) that acts as the precursor for an integrated network (or gel) of either discrete particles or network polymers. Typical precursors are metal alkoxides and metal chlorides, which undergo various forms of hydrolysis and polycondensation reactions.

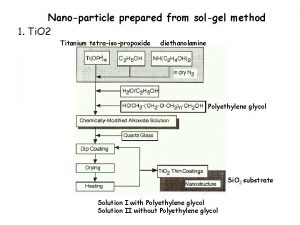
The sol gel method represents the low temperature way of the production of glasses, ceramic and composite materials with better purity and homogeneity than high temperature conventional processes. This process has been used to produce a wide range of compositions (mostly oxides) in various forms, including powders, organic/inorganic hybrids, fibers, coating, thin films, monoliths and porous membranes.


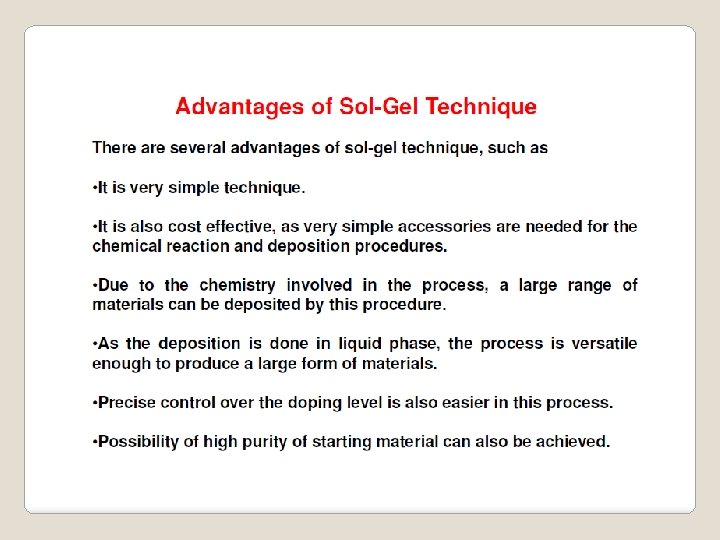
One of the most attractive features of the sol gel process is that it can produce compositions that cannot be created by the conventional methods. The mixing level of the solution is retained in the final product. In sol gel chemistry, the metal alkoxides convert to amorphous gels of metal oxides through hydrolysis and condensation reactions. The alkoxide solution route has been proven to be a good way to synthesis materials in different shapes such as films, fibers and powders.




Hydrothermal Synthesis

The term ‘hydrothermal’ came from the earth sciences, where it implied a regime of high temperatures and water pressures. Hydrothermal reactions: chemical processes at high pressures and temperatures over the boiling temperature in aqueous solutions For hydrothermal synthesis one needs a high temperature, highpressure apparatus called ‘autoclaves’ or ‘bombs’. Hydrothermal synthesis involves H 2 O at elevated temperature (>100 C) and high pressure (> a few atmosphere).

The water - as liquid or vapours – serves as the pressure transmitting medium. In addition, some or all of the reactants are partially soluble in the water under pressure and this enables reactions to take place in, or with the aid of, liquid and / or vapour phase. Hydrothermal methods have been used successfully for the synthesis of calcium silicate hydrates when lime and silica were heated in an autoclave at a temperature in the range 150 to 500 C and a pressure of 0. 1 to 2. 0 k bars.

The reactants are dissolved (or placed) in water or another solvent (solvothermal) in a closed vessel • Bomb is heated above BP • Conventional or MW oven • Commercially: – Tons of zeolites daily


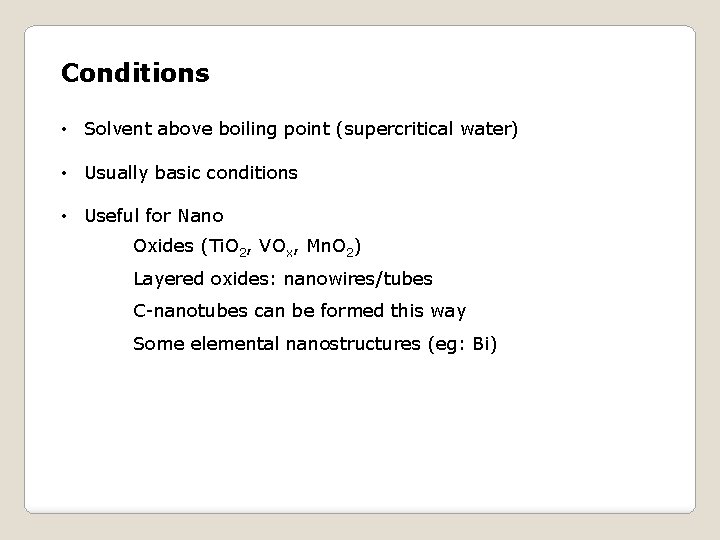
Conditions • Solvent above boiling point (supercritical water) • Usually basic conditions • Useful for Nano Oxides (Ti. O 2, VOx, Mn. O 2) Layered oxides: nanowires/tubes C-nanotubes can be formed this way Some elemental nanostructures (eg: Bi)

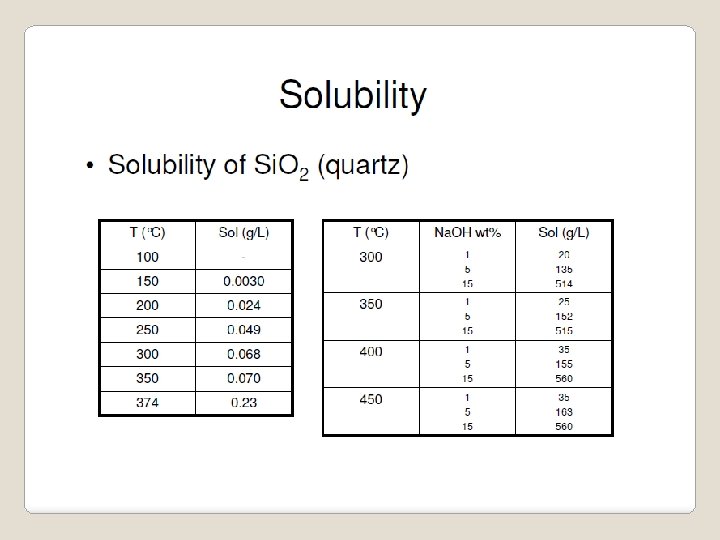
Mechanism • Usually follows a liquid nucleation model • Differs from solid-state-reaction mechanism from diffusion of atoms or ions between reactants • Due to enhanced solubility: Solubility of water increases with temperature, but alkaline solubility increases dramatically with temperature


 Solgel
Solgel Simple cubic unit cell
Simple cubic unit cell Hardness of nanomaterials
Hardness of nanomaterials Magnetic properties of nanomaterials
Magnetic properties of nanomaterials Solar energy diagram
Solar energy diagram Naturally occurring areas of hydrothermal resources
Naturally occurring areas of hydrothermal resources Naturally occurring areas of hydrothermal resources
Naturally occurring areas of hydrothermal resources Serpetinit
Serpetinit Miss holl
Miss holl Non foliated metamorphic rocks
Non foliated metamorphic rocks Chegg
Chegg Regional metamorphism
Regional metamorphism What are hydrothermal vent communities
What are hydrothermal vent communities Advantages and disadvantages of symposium
Advantages and disadvantages of symposium Synthesis matrix template
Synthesis matrix template Activation-synthesis theory
Activation-synthesis theory Whats a synthesis essay
Whats a synthesis essay Energeticism
Energeticism Vitamin k synthesis
Vitamin k synthesis