StateoftheART in Antiretroviral Management Joel E Gallant MD

![Slide 19 of 38 GS 104/111: TAF vs. TDF: Quantitative Proteinuria Urine [protein]: Creatinine Slide 19 of 38 GS 104/111: TAF vs. TDF: Quantitative Proteinuria Urine [protein]: Creatinine](https://slidetodoc.com/presentation_image_h/33411d602ef3212111a14c883b6bf854/image-4.jpg)
- Slides: 11

State-of-the-ART in Antiretroviral Management Joel E. Gallant, MD, MPH Medical Director, Specialty Services Southwest CARE Center Santa Fe, New Mexico FORMATTED: MM/DD/YY New Orleans, Louisiana: December 15 -17, 2015

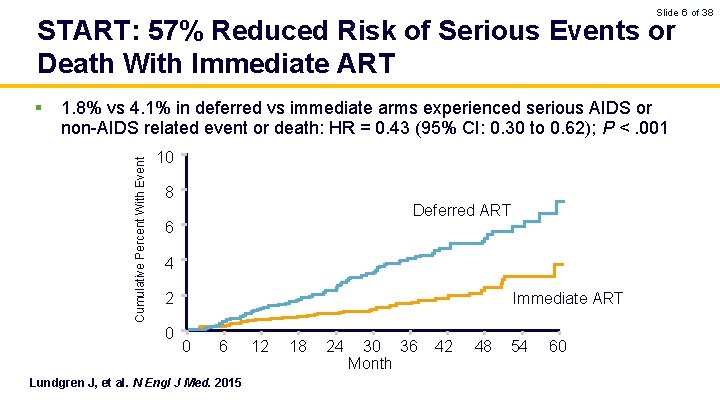
Slide 6 of 38 START: 57% Reduced Risk of Serious Events or Death With Immediate ART 1. 8% vs 4. 1% in deferred vs immediate arms experienced serious AIDS or non-AIDS related event or death: HR = 0. 43 (95% CI: 0. 30 to 0. 62); P <. 001 Cumulative Percent With Event § 10 8 Deferred ART 6 4 Immediate ART 2 0 0 6 Lundgren J, et al. N Engl J Med. 2015 12 18 24 30 36 Month 42 48 54 60

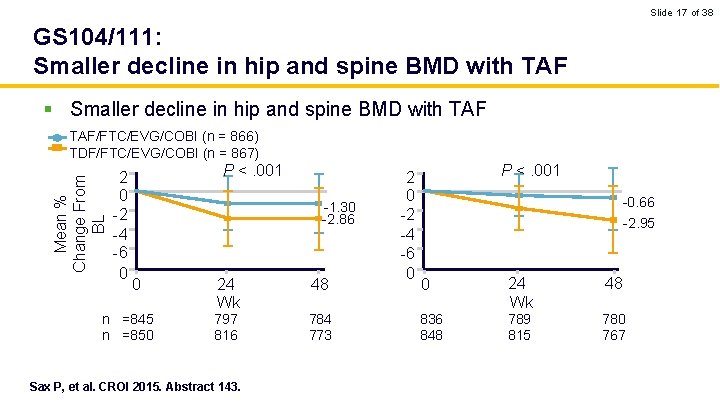
Slide 17 of 38 GS 104/111: Smaller decline in hip and spine BMD with TAF § Smaller decline in hip and spine BMD with TAF Mean % Change From BL TAF/FTC/EVG/COBI (n = 866) TDF/FTC/EVG/COBI (n = 867) 2 0 -2 -4 -6 0 P <. 001 -1. 30 -2. 86 0 n =845 n =850 24 Wk 797 816 Sax P, et al. CROI 2015. Abstract 143. 48 784 773 2 0 -2 -4 -6 0 P <. 001 -0. 66 -2. 95 0 836 848 24 Wk 789 815 48 780 767
![Slide 19 of 38 GS 104111 TAF vs TDF Quantitative Proteinuria Urine protein Creatinine Slide 19 of 38 GS 104/111: TAF vs. TDF: Quantitative Proteinuria Urine [protein]: Creatinine](https://slidetodoc.com/presentation_image_h/33411d602ef3212111a14c883b6bf854/image-4.jpg)
Slide 19 of 38 GS 104/111: TAF vs. TDF: Quantitative Proteinuria Urine [protein]: Creatinine Ratio Median % Change from Baseline (Q 1, Q 3) Protein (UPCR) Albumin (UACR) RBP 76 Beta 2 microglobulin 133 168 75 51 50 24 20 25 7 9 0 -25 -3 p <0. 001 for all -5 -32 -50 Baseline 44 mg/g E/C/F/TAF E/C/F/TDF 5 mg/g Sax P, et al. 22 nd CROI; Seattle, WA; February 23 -26, 2015. Abst. 143 LB. 64 μg/g 67 μg/g 101 μg/g 103 μg/g

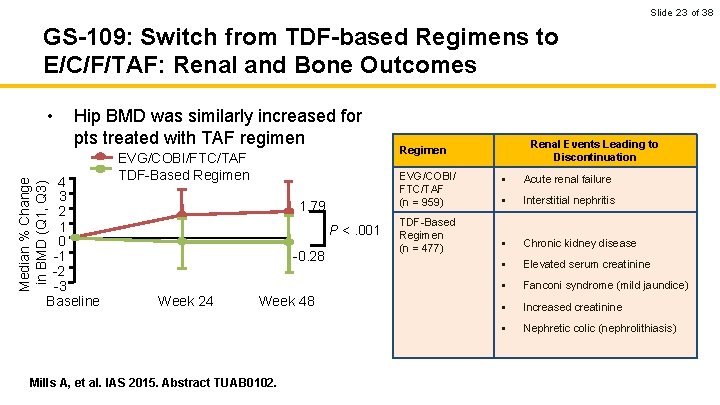
Slide 23 of 38 GS-109: Switch from TDF-based Regimens to E/C/F/TAF: Renal and Bone Outcomes • Hip BMD was similarly increased for pts treated with TAF regimen Median % Change in BMD (Q 1, Q 3) 4 3 2 1 0 -1 -2 -3 Baseline EVG/COBI/FTC/TAF TDF-Based Regimen EVG/COBI/ FTC/TAF (n = 959) 1. 79 P <. 001 -0. 28 Week 24 Week 48 Mills A, et al. IAS 2015. Abstract TUAB 0102. Renal Events Leading to Discontinuation Regimen TDF-Based Regimen (n = 477) § Acute renal failure § Interstitial nephritis § Chronic kidney disease § Elevated serum creatinine § Fanconi syndrome (mild jaundice) § Increased creatinine § Nephretic colic (nephrolithiasis)

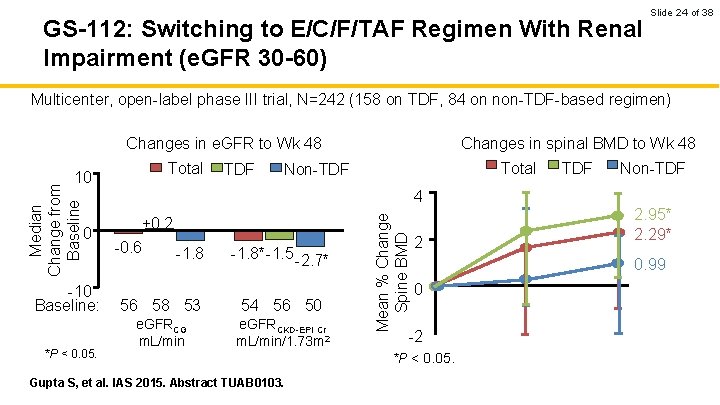
GS-112: Switching to E/C/F/TAF Regimen With Renal Impairment (e. GFR 30 -60) Slide 24 of 38 Multicenter, open-label phase III trial, N=242 (158 on TDF, 84 on non-TDF-based regimen) Changes in e. GFR to Wk 48 Total TDF Total Non-TDF 4 0 -10 Baseline: *P < 0. 05. +0. 2 -0. 6 -1. 8*-1. 5 -2. 7* 56 58 53 54 56 50 e. GFRCG m. L/min e. GFRCKD-EPI Cr m. L/min/1. 73 m 2 Gupta S, et al. IAS 2015. Abstract TUAB 0103. Mean % Change Spine BMD Median Change from Baseline 10 Changes in spinal BMD to Wk 48 2 2. 95* 2. 29* 0. 99 0 -2 *P < 0. 05.

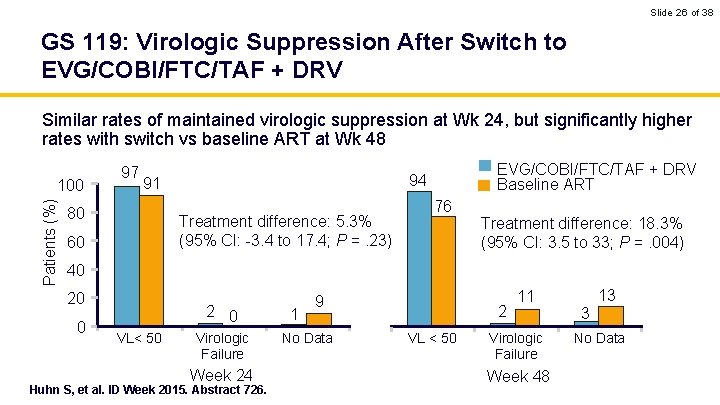
Slide 26 of 38 GS 119: Virologic Suppression After Switch to EVG/COBI/FTC/TAF + DRV Similar rates of maintained virologic suppression at Wk 24, but significantly higher rates with switch vs baseline ART at Wk 48 Patients (%) 100 97 91 80 Treatment difference: 5. 3% (95% CI: -3. 4 to 17. 4; P =. 23) 60 EVG/COBI/FTC/TAF + DRV Baseline ART 94 76 Treatment difference: 18. 3% (95% CI: 3. 5 to 33; P =. 004) 40 20 0 2 0 VL< 50 Virologic Failure Week 24 Huhn S, et al. ID Week 2015. Abstract 726. 1 9 No Data 2 VL < 50 11 Virologic Failure Week 48 13 3 No Data

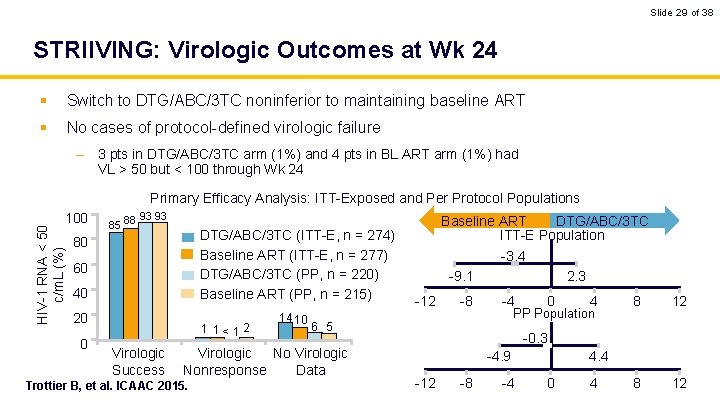
Slide 29 of 38 STRIIVING: Virologic Outcomes at Wk 24 § Switch to DTG/ABC/3 TC noninferior to maintaining baseline ART § No cases of protocol-defined virologic failure – 3 pts in DTG/ABC/3 TC arm (1%) and 4 pts in BL ART arm (1%) had VL > 50 but < 100 through Wk 24 Primary Efficacy Analysis: ITT-Exposed and Per Protocol Populations HIV-1 RNA < 50 c/m. L (%) 100 85 88 93 93 DTG/ABC/3 TC (ITT-E, n = 274) Baseline ART (ITT-E, n = 277) DTG/ABC/3 TC (PP, n = 220) Baseline ART (PP, n = 215) 80 60 40 20 0 1 1<12 Virologic Success 14 10 -3. 4 -9. 1 -12 -8 2. 3 -4 0 4 PP Population 6 5 Virologic Nonresponse Data Trottier B, et al. ICAAC 2015. Baseline ART DTG/ABC/3 TC ITT-E Population 8 12 -0. 3 -4. 9 -12 -8 -4 4. 4 0 4

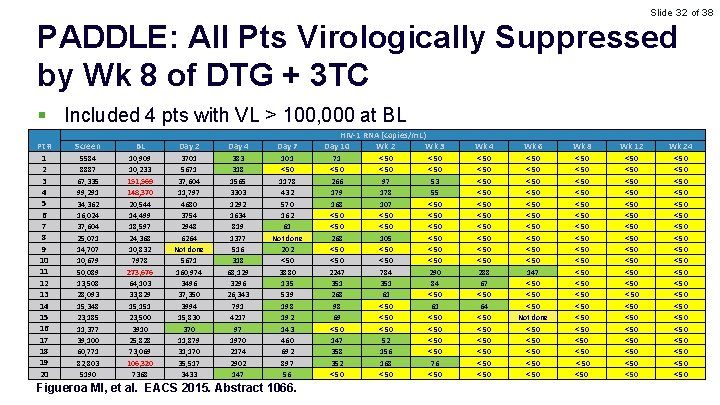
Slide 32 of 38 PADDLE: All Pts Virologically Suppressed by Wk 8 of DTG + 3 TC § Included 4 pts with VL > 100, 000 at BL Pt # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Screen BL Day 2 Day 4 Day 7 5584 8887 67, 335 99, 291 34, 362 16, 024 37, 604 25, 071 14, 707 10, 679 50, 089 13, 508 28, 093 15, 348 23, 185 11, 377 39, 100 60, 771 82, 803 5190 10, 909 10, 233 151, 569 148, 370 20, 544 14, 499 18, 597 24, 368 10, 832 7978 273, 676 64, 103 33, 829 15, 151 23, 500 3910 25, 828 73, 069 106, 320 7368 3701 5671 37, 604 11, 797 4680 3754 2948 6264 Not done 5671 160, 974 3496 37, 350 3994 15, 830 370 11, 879 31, 170 35, 517 3433 383 318 1565 3303 1292 1634 819 1377 516 318 68, 129 3296 26, 343 791 4217 97 1970 2174 2902 147 101 < 50 1178 432 570 162 61 Not done 202 < 50 3880 135 539 198 192 143 460 692 897 56 Figueroa MI, et al. EACS 2015. Abstract 1066. HIV-1 RNA (copies/m. L) Day 10 Wk 2 Wk 3 71 < 50 266 179 168 < 50 2247 351 268 98 69 < 50 147 358 352 < 50 97 178 107 < 50 105 < 50 784 351 61 < 50 52 156 168 < 50 53 55 < 50 < 50 290 84 < 50 61 < 50 76 < 50 Wk 4 Wk 6 Wk 8 Wk 12 Wk 24 < 50 < 50 < 50 288 67 < 50 64 < 50 < 50 < 50 < 50 147 < 50 Not done < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50 < 50

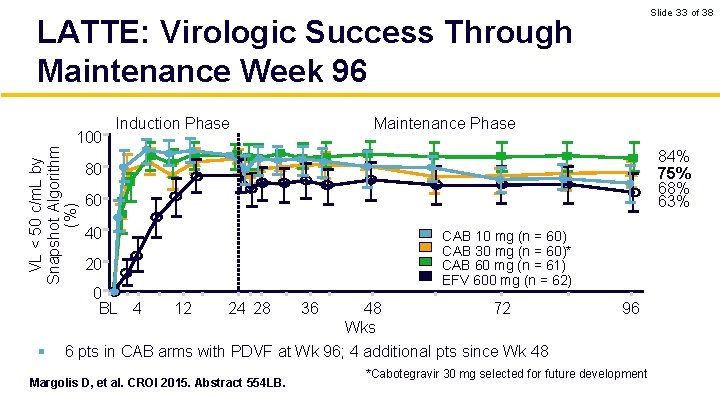
Slide 33 of 38 LATTE: Virologic Success Through Maintenance Week 96 VL < 50 c/m. L by Snapshot Algorithm (%) 100 § Induction Phase Maintenance Phase 84% 75% 68% 63% 80 60 40 CAB 10 mg (n = 60) CAB 30 mg (n = 60)* CAB 60 mg (n = 61) EFV 600 mg (n = 62) 20 0 BL 4 12 24 28 48 72 Wks 6 pts in CAB arms with PDVF at Wk 96; 4 additional pts since Wk 48 Margolis D, et al. CROI 2015. Abstract 554 LB. 36 96 *Cabotegravir 30 mg selected for future development

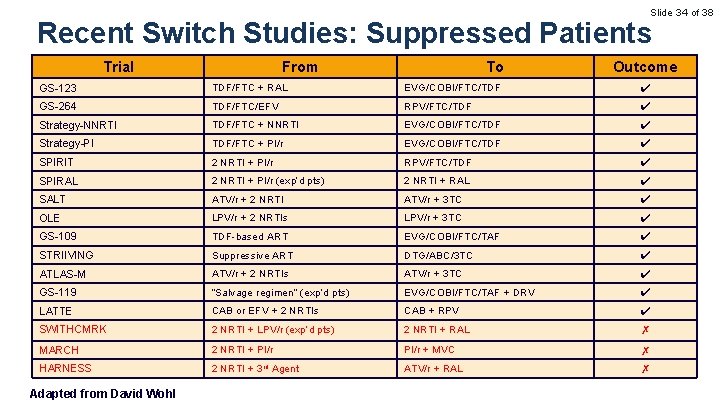
Slide 34 of 38 Recent Switch Studies: Suppressed Patients Trial From To Outcome GS-123 TDF/FTC + RAL EVG/COBI/FTC/TDF ✔ GS-264 TDF/FTC/EFV RPV/FTC/TDF ✔ Strategy-NNRTI TDF/FTC + NNRTI EVG/COBI/FTC/TDF ✔ Strategy-PI TDF/FTC + PI/r EVG/COBI/FTC/TDF ✔ SPIRIT 2 NRTI + PI/r RPV/FTC/TDF ✔ SPIRAL 2 NRTI + PI/r (exp’d pts) 2 NRTI + RAL ✔ SALT ATV/r + 2 NRTI ATV/r + 3 TC ✔ OLE LPV/r + 2 NRTIs LPV/r + 3 TC ✔ GS-109 TDF-based ART EVG/COBI/FTC/TAF ✔ STRIIVING Suppressive ART DTG/ABC/3 TC ✔ ATLAS-M ATV/r + 2 NRTIs ATV/r + 3 TC ✔ GS-119 “Salvage regimen” (exp’d pts) EVG/COBI/FTC/TAF + DRV ✔ LATTE CAB or EFV + 2 NRTIs CAB + RPV ✔ SWITHCMRK 2 NRTI + LPV/r (exp’d pts) 2 NRTI + RAL ✗ MARCH 2 NRTI + PI/r + MVC ✗ HARNESS 2 NRTI + 3 rd Agent ATV/r + RAL ✗ Adapted from David Wohl





















