Research Services Research ethics and personalsensitive data at














- Slides: 16

Research Services Research ethics and personal/sensitive data at Oxford Social Sciences and Humanities IDREC Claudia Kozeny-Pelling, Research Ethics Manager ethics@socsci. ox. ac. uk Michaelmas term 2016 www. admin. ox. ac. uk/curec

Research Services Governing ethics and conduct “The University requires that all those carrying out such research engage with the University’s commitment to conduct research to high ethical standards; understand the reasons for, and participate fully in, the ethical review process. ” University policy on the ethical conduct of research involving human participants and personal data applies to all research conducted by members of the University since 2006 Ethics / Integrity Policy Also see guidelines by professional associations Research Ethics Committees Researcher integrity Academic integrity in research: Code of practice and procedure

Research Services The Concordat to Support Research Integrity Maintaining the highest standards of research integrity Researchers will: • ensure that all research is subject to active and appropriate consideration of ethical issues • comply with ethical, legal and professional frameworks, obligations and standards as required by statutory and regulatory authorities, and by employers, funders and other relevant stakeholders Employers of researchers are responsible for: • having clear policies on ethical approval available to all researchers • making sure that all researchers are aware of and understand policies and processes relating to ethical approval

Research Services University Policy on the Ethical Conduct of Research Involving Human Participants and Personal Data Extract. . • The University is committed to ensuring that research involving human participants and personal data conducted on University premises or using University facilities or by University researchers is carried out to high ethical standards • The University meets this commitment by • identifying and reviewing all research involving human participants and personal data in proportion to the level of risk, except where the ethical standards of that research are more appropriately secured by another recognised approval procedure, for example that of the National Health Service. • www. admin. ox. ac. uk/curec/policystatement/ - applies to all research conducted by members of the University of Oxford since October 2006

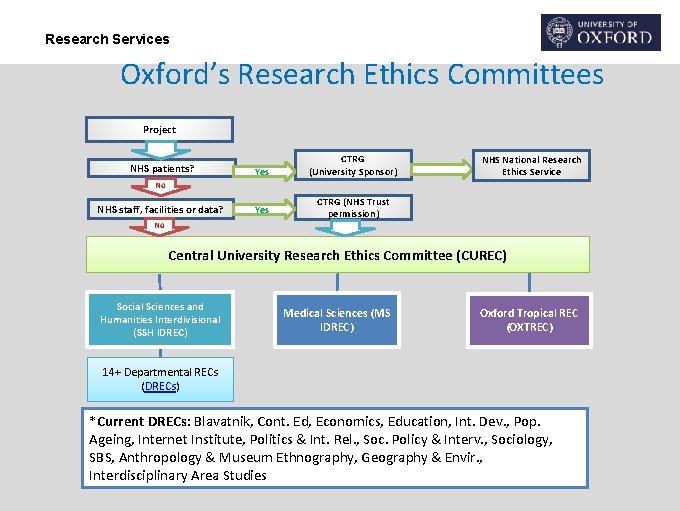
Research Services Oxford’s Research Ethics Committees Project NHS patients? Yes CTRG (University Sponsor) Yes CTRG (NHS Trust permission) NHS National Research Ethics Service No NHS staff, facilities or data? No Central University Research Ethics Committee (CUREC) Social Sciences and Humanities Interdivisional (SSH IDREC) Medical Sciences (MS IDREC) Oxford Tropical REC (OXTREC) 14+ Departmental RECs (DRECs) *Current DRECs: Blavatnik, Cont. Ed, Economics, Education, Int. Dev. , Pop. Ageing, Internet Institute, Politics & Int. Rel. , Soc. Policy & Interv. , Sociology, SBS, Anthropology & Museum Ethnography, Geography & Envir. , Interdisciplinary Area Studies

Research Services What needs CUREC review? • Fresh data collection (even if you de-identify/anonymise data later) • Generalisable, generally applicable enquiry/results • Any projects for (open-access) publication

Research Services Personal data • “…Data which relate to a living individual who can be identified from those data or from those data and other information which is in the possession of, or is likely to come into the possession of the data controller and includes any expression of opinion about the individual… this includes any other person in respect of the individual”. (Data Protection Act 1998). • Researchers must be clear from the outset about why they are collecting personal data and what they intend to do with it. • Personal data must be deleted once it is no longer required for the study. • Personal data of children under 18: researchers need explicit and verifiable consent of the child’s parent or guardian and the child’s assent. Child’s lack of assent will always overrule the parent’s or guardian’s consent. See CUREC-Approved Procedure 15 or Approved Procedure 25 for common research studies with children at schools or non-institutional settings, including informed consent templates.

Research Services Sensitive personal data • Racial or ethnic origins • Political beliefs • Religious or other beliefs • Trade union membership • Health • Sex life • Criminal allegations, proceedings or convictions If the researcher needs to capture sensitive personal data, explicit consent for this is required.

Research Services Informed consent and personal / sensitive • Ensure that written/oral consent process complies with Data Protection Act and data the University Policy on the Management of Research Data and Records • Explain who will process the data, and how it will be processed (e. g. encryption) • Who else will see the data (will they be outside of the European Economic Area? ) • Purpose of the study / data collection • Participants’ rights • What happens to the personal data and research data at the end of the project? How long will research data be stored (see also University Policy on the Management of Research Data and Records) • Gain explicit consent for collecting and processing any sensitive data. “A combination of gaining consent for data sharing, anonymising data and controlling access to data can enable the ethical and legal sharing of data. ” (UK Data Archive)

Research Services Sharing and re-using personal/sensitive data • Obtain informed consent, also for data sharing, preservation, curation, re-use • Protect identities e. g. anonymization • Restrict / regulate access where needed (all or part of data) e. g. by group, use, time period • Securely store personal or sensitive data (check Researchdata website and contact researchdata@ox. ac. uk for help) • Anonymised research data should be kept for a minimum of three years after publication, or longer depending on funder requirements (e. g. ESRC, Wellcome Trust, MRC…). Contact researchdata@ox. ac. uk for advice.

Research Services Informed consent templates to adapt, if appropriate • For online surveys: see Appendix A of the Best Practice Guidance on Internet. Based Research at www. admin. ox. ac. uk/curec/resources/bestpractice/ • Written consent information sheet and consent templates: www. admin. ox. ac. uk/curec/resources/informed-consent/#d. en. 243079 • Oral consent script template (for some Social Sciences/Humanities and some Ox. TREC studies, if written consent is not appropriate): www. admin. ox. ac. uk/curec/resources/informed-consent/#d. en. 243079 • More about informed consent: www. admin. ox. ac. uk/curec/resources/informed-consent • Some of CUREC’s Approved Procedures have their own informed consent documents researchers will need to adapt. www. admin. ox. ac. uk/curec/resources/protocols/

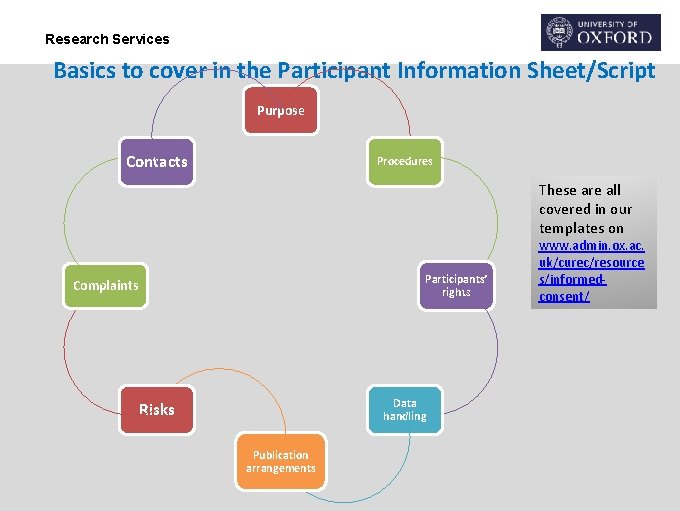
Research Services Basics to cover in the Participant Information Sheet/Script Purpose Contacts Procedures These are all covered in our templates on Participants’ rights Complaints Data handling Risks Publication arrangements www. admin. ox. ac. uk/curec/resource s/informedconsent/

Research Services The Consent form: Participant understands… Purpose of Study Contents of Participant Information Sheet Study has CUREC Ethics Approval Right to Withdraw Data Handling and Publication Plans Risks How to Raise a Concern / Complain Consent to being photographed / audio / video recorded / to extract DNA (if applicable) Consent to Study Participation These are all covered in our templates: www. admin. ox. ac. uk/curec /resources/informedconsent/

Research Services How to apply for ethical review See www. admin. ox. ac. uk/curec/apply/ • • “Straightforward issues” application (CUREC 1 A checklist and supporting documents) Full “Complex issues” application (CUREC 2 form and supporting documents) CUREC 2 may be needed if: • No consent • Severe deception • High participant risk • Sensitive topics (abuse), criminal activity • Other legal breaches (data) • Not fully covered by one ‘Approved Procedure’ Review timeframes differ! Straightforward (CUREC 1 or 1 A) = 30 days Complex (CUREC 2) = 60 days Drugs (CUREC 3) = 90 days [MS IDREC only]

Research Services Useful websites CUREC website: www. admin. ox. ac. uk/curec/ Oxford Research Data website: http: //researchdata. ox. ac. uk/ UK Data Archive: www. data-archive. ac. uk/ especially www. data-archive. ac. uk/create-manage/consent-ethics

Research Services Questions about research ethics at Oxford? Social Sciences and Humanities IDREC Secretariat: ethics@socsci. ox. ac. uk (Claudia Kozeny-Pelling and Justine Malone) The SSH IDREC also deals with some departments in MPLS. Departmental Research Ethics Committees (DRECs): www. admin. ox. ac. uk/curec/about/drecs Medical Sciences IDREC Secretariat: ethics@medsci. ox. ac. uk (Helen Barnby-Porritt and Leah Butts) Oxford Tropical Medicine IDREC Secretariat: oxtrec@admin. ox. ac. uk (Rebecca Bryant and Sami Kelsh) www. admin. ox. ac. uk/curec/contacts/