PROCESS VALIDATION OF VARIOUS FORMULATIONS PART3 PROCESS VALIDATION










- Slides: 13

PROCESS VALIDATION OF VARIOUS FORMULATIONS PART-3 PROCESS VALIDATION OF CAPSULES PHARMACEUTICAL QUALITY ASSURANCE DEPARTMENT OF PHARMACEUTICAL SCIENCES & TECHNOLOGY BIRLA INSTITUTE OF TECHNOLOGY, MESRA

LEARNING OUTCOME • After watching this Video lecture we are going to LEARN: • WHAT IS PROCESS VALIDATION? • WHAT ARE THE PARAMETERS OF PROCESS VALIDATION OF CAPSULES?

What is Process Validation?

WHAT IS PROCESS VALIDATION? PROCESS VALIDATION is a means of ensuring that manufacturing processes are capable of consistently producing a finished product of the required quality. Effective process validation significantly provides assurance of the drug quality. The principle of this process incorporates the understanding that the following conditions exist: • Quality, safety, and efficacy are designed or built into the product. • Quality cannot be adequately assured merely by in-process and finished-product inspection or testing.

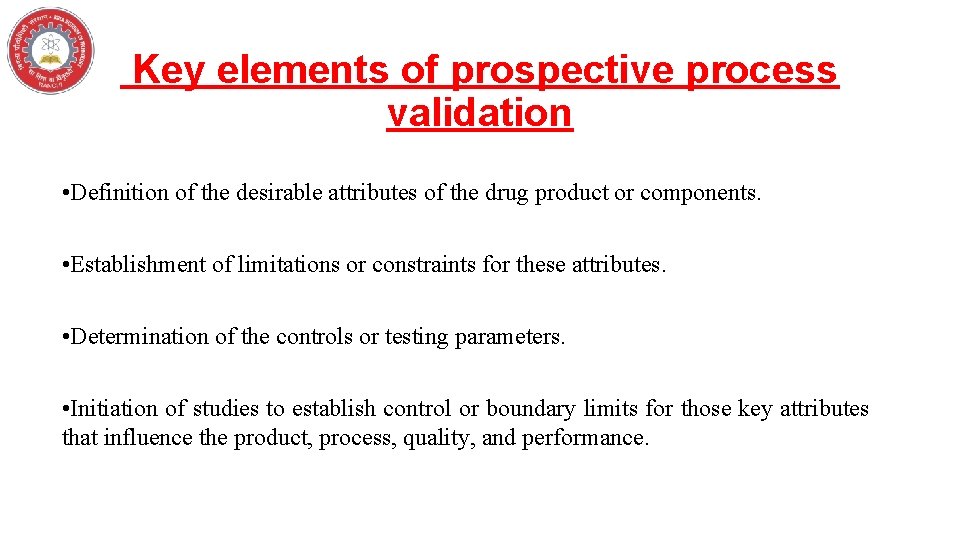
Key elements of prospective process validation

Key elements of prospective process validation • Definition of the desirable attributes of the drug product or components. • Establishment of limitations or constraints for these attributes. • Determination of the controls or testing parameters. • Initiation of studies to establish control or boundary limits for those key attributes that influence the product, process, quality, and performance.

• Now, please take a pause for 2 minutes to recapitulate the topics we have discussed, and try to answer the following questions: • What is Process Validation? • What are the Key Elements of Prospective Process Validation?

Process Validation Of Capsules

Validation of raw material • Raw materials include API, Gelatin, Colorant, Sugar, Water, Sulfur-dioxide, Preservative, Diluents, Lubricant, Wetting agent, Disintegrates, etc. • Justification of their use. • Justification for the amount. • Each raw material should be validated by performing checks on several batches. • Stability of the raw material should be assessed. • The final step of raw material validation should involve an on-site inspection of the supplier.

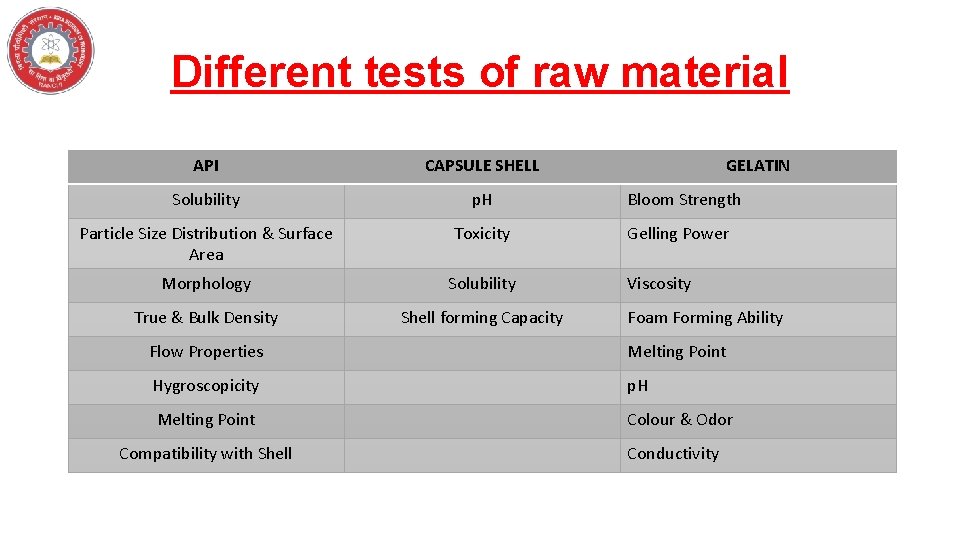
Different tests of raw material API CAPSULE SHELL Solubility p. H Particle Size Distribution & Surface Area Toxicity Morphology Solubility True & Bulk Density Shell forming Capacity GELATIN Bloom Strength Gelling Power Viscosity Foam Forming Ability Flow Properties Melting Point Hygroscopicity p. H Melting Point Colour & Odor Compatibility with Shell Conductivity

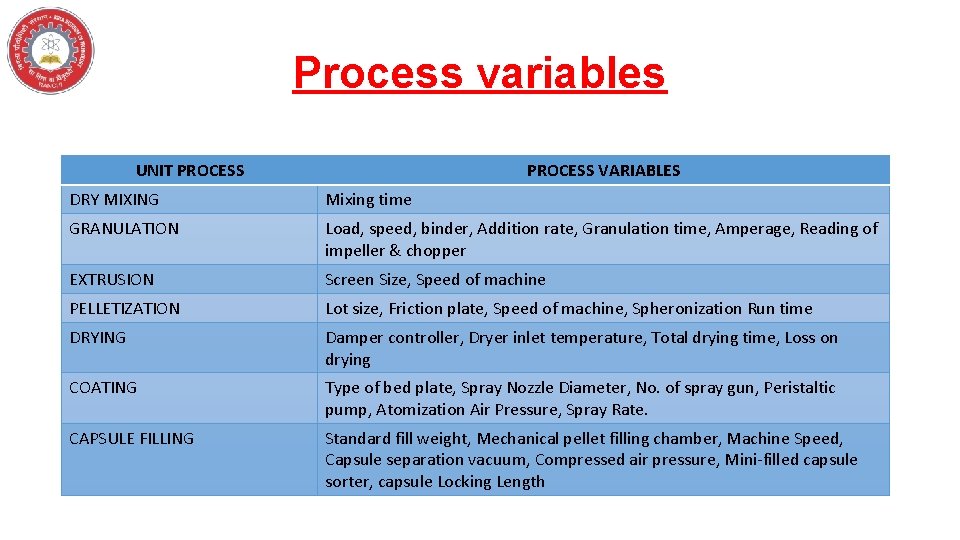
Process variables UNIT PROCESS VARIABLES DRY MIXING Mixing time GRANULATION Load, speed, binder, Addition rate, Granulation time, Amperage, Reading of impeller & chopper EXTRUSION Screen Size, Speed of machine PELLETIZATION Lot size, Friction plate, Speed of machine, Spheronization Run time DRYING Damper controller, Dryer inlet temperature, Total drying time, Loss on drying COATING Type of bed plate, Spray Nozzle Diameter, No. of spray gun, Peristaltic pump, Atomization Air Pressure, Spray Rate. CAPSULE FILLING Standard fill weight, Mechanical pellet filling chamber, Machine Speed, Capsule separation vacuum, Compressed air pressure, Mini-filled capsule sorter, capsule Locking Length

• IN THE NEXT VIDEO WE ARE GOING TO LEARN ABOUT METHOD VALIDATION OF OINTMENTS AND CREAMS.

Prepared by: Ms. Snigdha Baag Ms. Riya Banerjee Dr. Kishanta K. Pradhan Dr. Manik Ghosh PHARMACEUTICAL QUALITY ASSURANCE DEPARTMENT OF PHARMACEUTICAL SCIENCES & TECHNOLOGY BIRLA INSTITUTE OF TECHNOLOGY, MESRA