Physical and Chemical Changes A Matter Flowchart MATTER







- Slides: 15

Physical and Chemical Changes

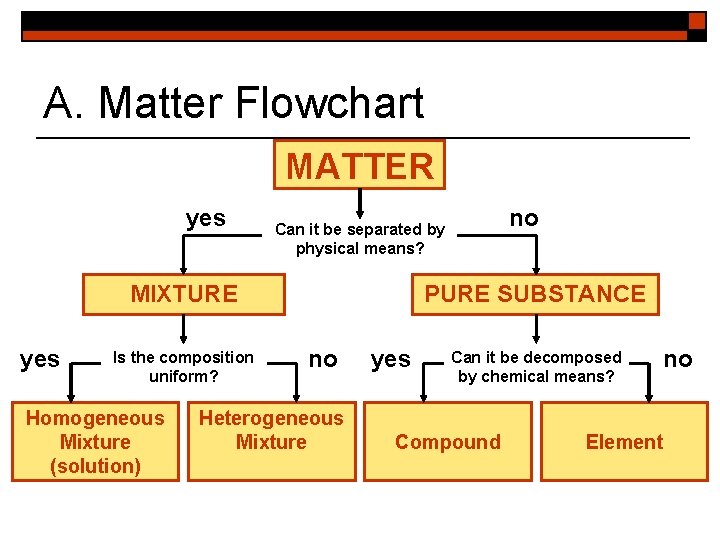
A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Can it be separated by physical means? PURE SUBSTANCE no Heterogeneous Mixture yes Can it be decomposed by chemical means? Compound no Element

B. Pure Substances o Element n n matter composed of identical atoms EX: copper

B. Pure Substances o Compound n n n matter composed of 2 or more elements in a fixed ratio properties differ from those of individual elements EX: salt (Na. Cl)

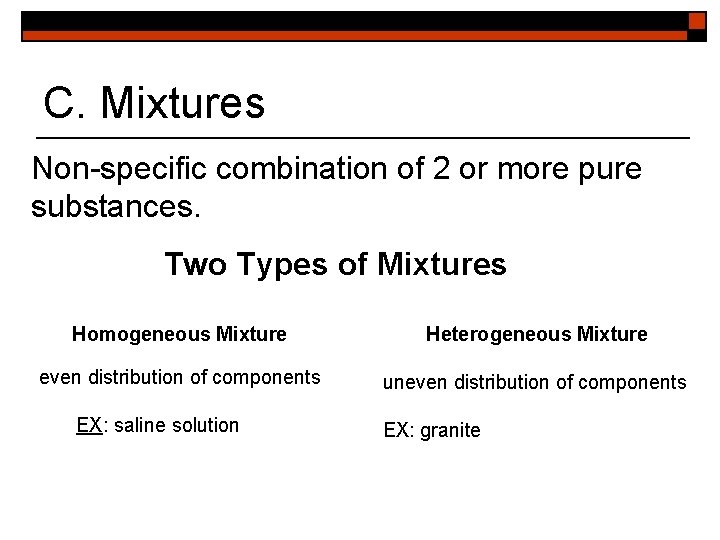
C. Mixtures Non-specific combination of 2 or more pure substances. Two Types of Mixtures Homogeneous Mixture Heterogeneous Mixture even distribution of components uneven distribution of components EX: saline solution EX: granite

A. Physical Property o A characteristic of matter that can be observed or measured without changing the identity of its matter. n can be used to separate mixtures n EX: density, color, odor, mass and volume

Chemical Property o A characteristic that indicates whether a substance can undergo a specific chemical change. n EX: flammability, reactivity

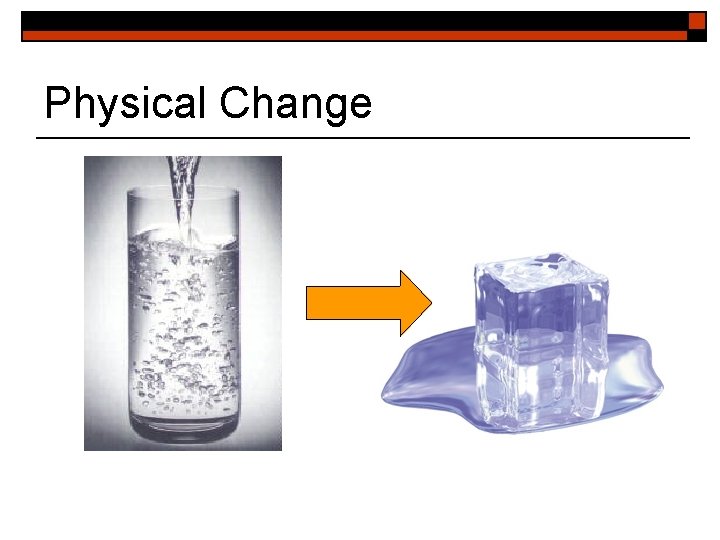
Physical Change o o Physical changes occur when matter changes its property but not its chemical nature. Physical changes could include a change in: texture, shape, size, color, odor, volume, mass, weight, and density.

Physical Change Remember! n properties remain the same n Reversible (most of the time) n can be used to separate mixtures

Physical Change

Chemical Change o o Chemical changes are changes matter undergoes when it becomes new or different matter. To identify a chemical change look for signs such as color change, bubbling and fizzing, light production, smoke, and presence of heat.

C. Chemical Change Remember! n properties change n Irreversible (most of the time, at least without going through a chemical process)

Possible Clues a chemical Reaction has taken place! 1. 2. 3. 4. 5. 6. 7. Change in color Change in state Change in shape Gets cold (endothermic) Gets hot (exothermic) Gas created (bubbles/fizzing) Gives off light

Possible Clues a chemical Reaction has taken place! Gives off a sound (explosion) 9. Gives off a smell (spoiling/cooking) 10. Precipitate forms (two liquids are mixed and a solid is created) 8.

Chemical Change o A chemical change occurs when fireworks are used. Fireworks are made of metals such as magnesium and copper. These change chemically as they light up the sky.