PGx Logical Overview PGx Alert Guideline Development PGx

- Slides: 5

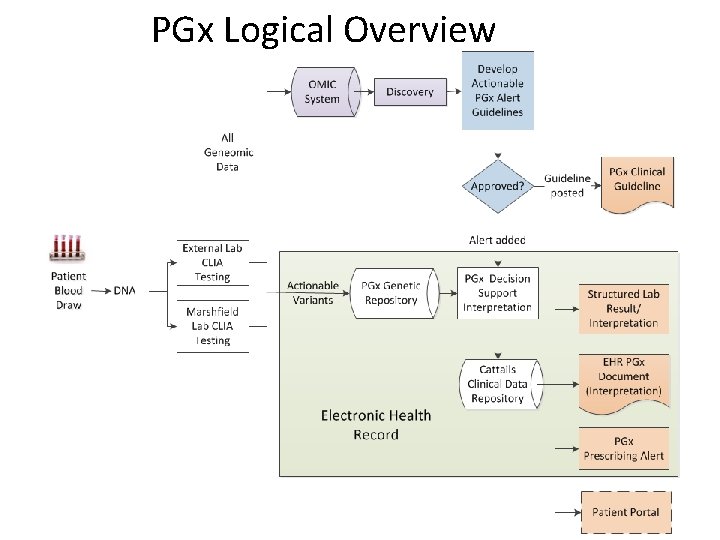
PGx Logical Overview

PGx Alert & Guideline Development • PGx Committee – Clinical Geneticist – Pharmacists – Physicians and Specialists – Lab Director – Quality Director

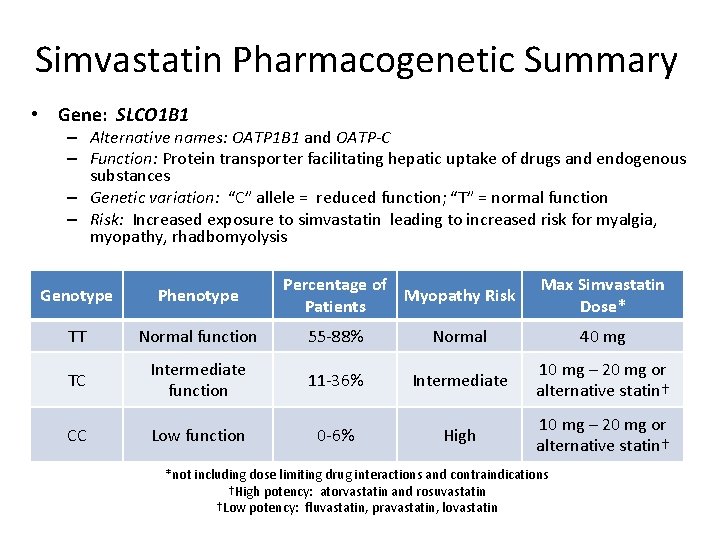
Simvastatin Pharmacogenetic Summary • Gene: SLCO 1 B 1 – Alternative names: OATP 1 B 1 and OATP-C – Function: Protein transporter facilitating hepatic uptake of drugs and endogenous substances – Genetic variation: “C” allele = reduced function; “T” = normal function – Risk: Increased exposure to simvastatin leading to increased risk for myalgia, myopathy, rhadbomyolysis Percentage of Myopathy Risk Patients Max Simvastatin Dose* Genotype Phenotype TT Normal function 55 -88% Normal 40 mg TC Intermediate function 11 -36% Intermediate 10 mg – 20 mg or alternative statin† CC Low function 0 -6% High 10 mg – 20 mg or alternative statin† *not including dose limiting drug interactions and contraindications †High potency: atorvastatin and rosuvastatin †Low potency: fluvastatin, pravastatin, lovastatin

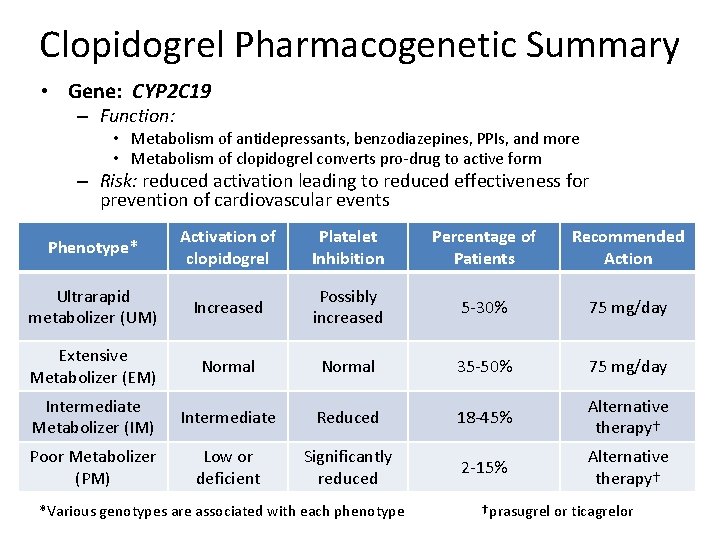
Clopidogrel Pharmacogenetic Summary • Gene: CYP 2 C 19 – Function: • Metabolism of antidepressants, benzodiazepines, PPIs, and more • Metabolism of clopidogrel converts pro-drug to active form – Risk: reduced activation leading to reduced effectiveness for prevention of cardiovascular events Phenotype* Activation of clopidogrel Platelet Inhibition Percentage of Patients Recommended Action Ultrarapid metabolizer (UM) Increased Possibly increased 5 -30% 75 mg/day Extensive Metabolizer (EM) Normal 35 -50% 75 mg/day Intermediate Metabolizer (IM) Intermediate Reduced 18 -45% Alternative therapy† Poor Metabolizer (PM) Low or deficient Significantly reduced 2 -15% Alternative therapy† *Various genotypes are associated with each phenotype †prasugrel or ticagrelor

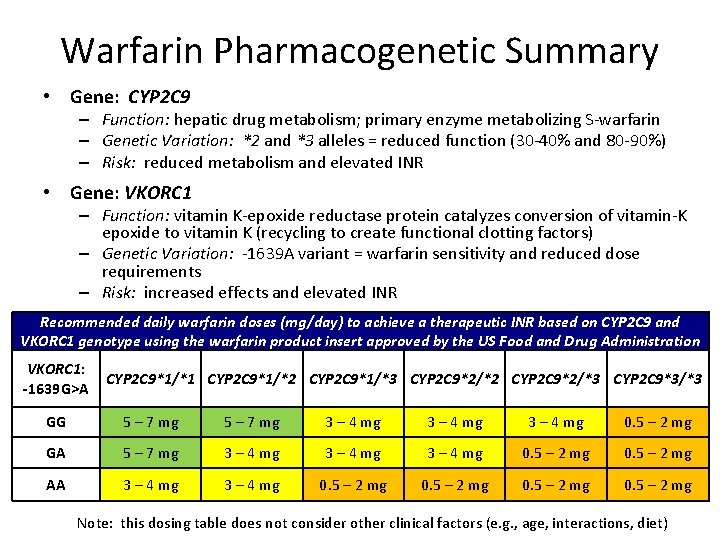
Warfarin Pharmacogenetic Summary • Gene: CYP 2 C 9 – Function: hepatic drug metabolism; primary enzyme metabolizing S-warfarin – Genetic Variation: *2 and *3 alleles = reduced function (30 -40% and 80 -90%) – Risk: reduced metabolism and elevated INR • Gene: VKORC 1 – Function: vitamin K-epoxide reductase protein catalyzes conversion of vitamin-K epoxide to vitamin K (recycling to create functional clotting factors) – Genetic Variation: -1639 A variant = warfarin sensitivity and reduced dose requirements – Risk: increased effects and elevated INR Recommended daily warfarin doses (mg/day) to achieve a therapeutic INR based on CYP 2 C 9 and VKORC 1 genotype using the warfarin product insert approved by the US Food and Drug Administration VKORC 1: -1639 G>A CYP 2 C 9*1/*1 CYP 2 C 9*1/*2 CYP 2 C 9*1/*3 CYP 2 C 9*2/*2 CYP 2 C 9*2/*3 CYP 2 C 9*3/*3 GG 5 – 7 mg 3 – 4 mg 0. 5 – 2 mg GA 5 – 7 mg 3 – 4 mg 0. 5 – 2 mg AA 3 – 4 mg 0. 5 – 2 mg Note: this dosing table does not consider other clinical factors (e. g. , age, interactions, diet)