Perhaps one of you gentlemen would mind telling
- Slides: 7

“Perhaps one of you gentlemen would mind telling me just what is outside the window that you find so attractive. . ? ” Image courtesy Nearing. Zero. net

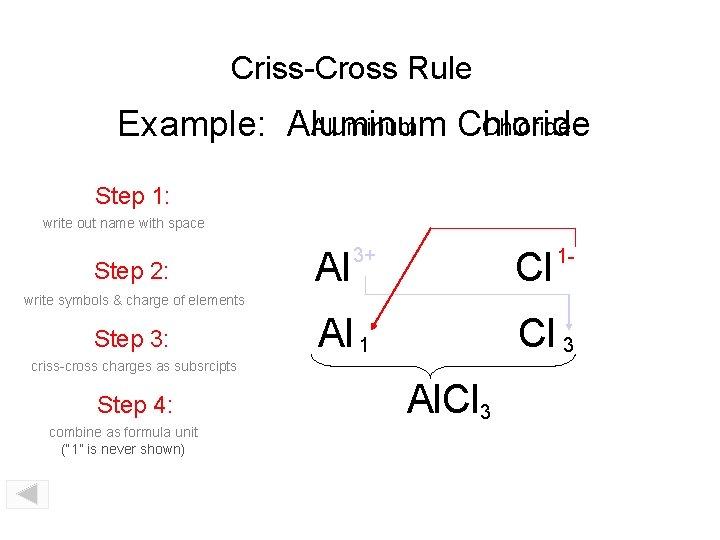
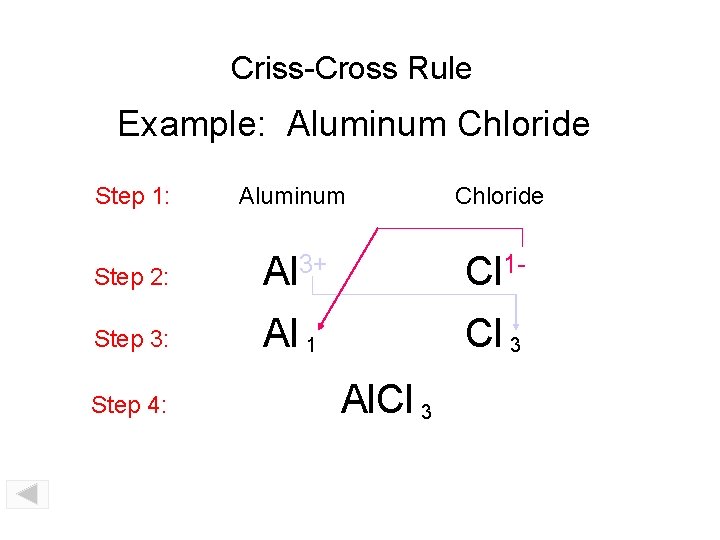
Criss-Cross Rule Aluminum Chloride Example: Aluminum Chloride Step 1: write out name with space Step 2: Al 3+ Cl 1 - write symbols & charge of elements Step 3: Al 1 Cl 3 criss-cross charges as subsrcipts Step 4: combine as formula unit (“ 1” is never shown) Al. Cl 3

Criss-Cross Rule Example: Aluminum Chloride Step 1: Aluminum Chloride Step 2: Al 3+ Cl 1 - Step 3: Al 1 Cl 3 Step 4: Al. Cl 3

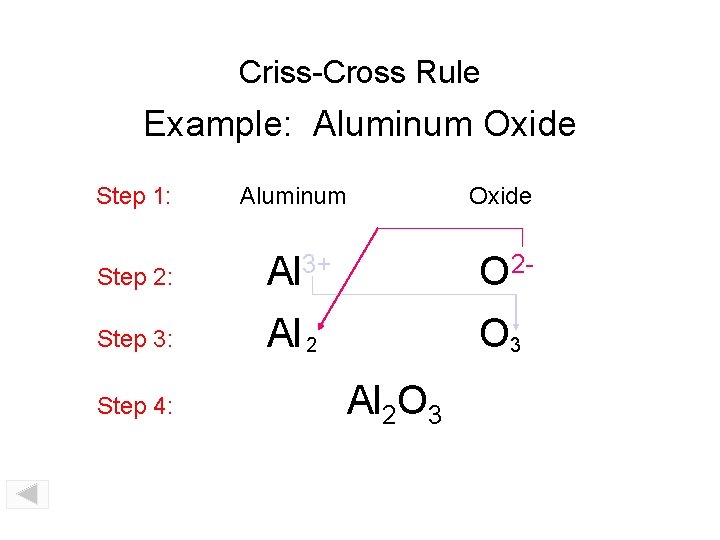
Criss-Cross Rule Example: Aluminum Oxide Step 1: Aluminum Oxide Step 2: Al 3+ O 2 - Step 3: Al 2 O 3 Step 4: Al 2 O 3

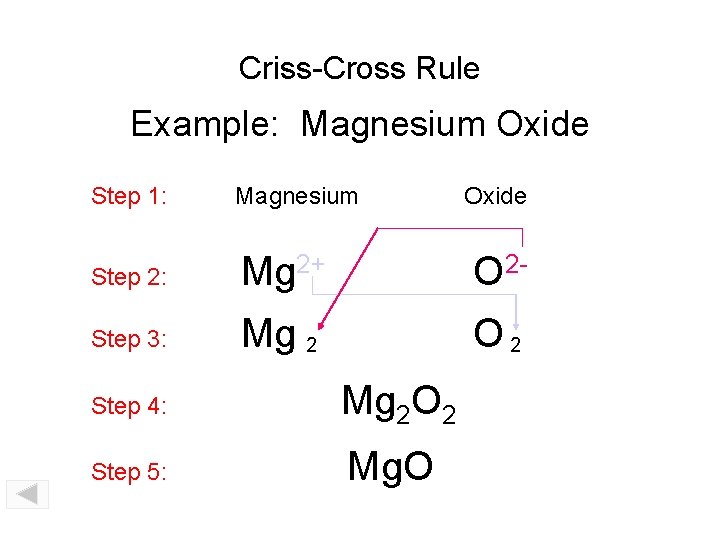
Criss-Cross Rule Example: Magnesium Oxide Step 1: Magnesium Step 2: Mg 2+ O 2 - Step 3: Mg 2 O 2 Step 4: Mg 2 O 2 Step 5: Mg. O Oxide

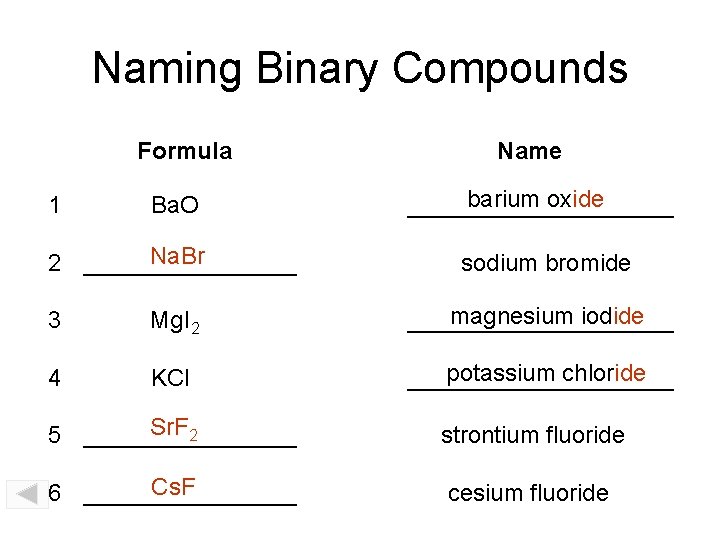
Naming Binary Compounds Formula Name Ba. O barium oxide __________ Na. Br 2 ________ sodium bromide 1 3 Mg. I 2 magnesium iodide __________ 4 KCl potassium chloride __________ Sr. F 2 5 ________ strontium fluoride Cs. F 6 ________ cesium fluoride

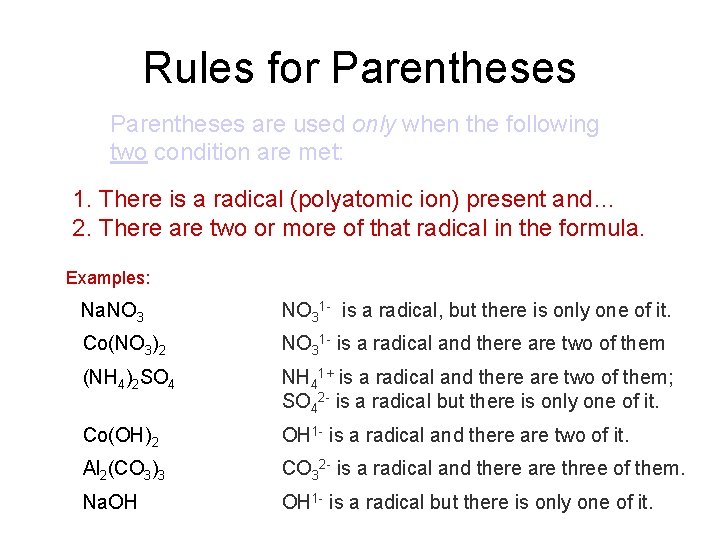
Rules for Parentheses are used only when the following two condition are met: 1. There is a radical (polyatomic ion) present and… 2. There are two or more of that radical in the formula. Examples: Na. NO 31 - is a radical, but there is only one of it. Co(NO 3)2 NO 31 - is a radical and there are two of them (NH 4)2 SO 4 NH 41+ is a radical and there are two of them; SO 42 - is a radical but there is only one of it. Co(OH)2 OH 1 - is a radical and there are two of it. Al 2(CO 3)3 CO 32 - is a radical and there are three of them. Na. OH OH 1 - is a radical but there is only one of it.












