Performance of Legiolert for Quantification of Legionella pneumophila
- Slides: 1

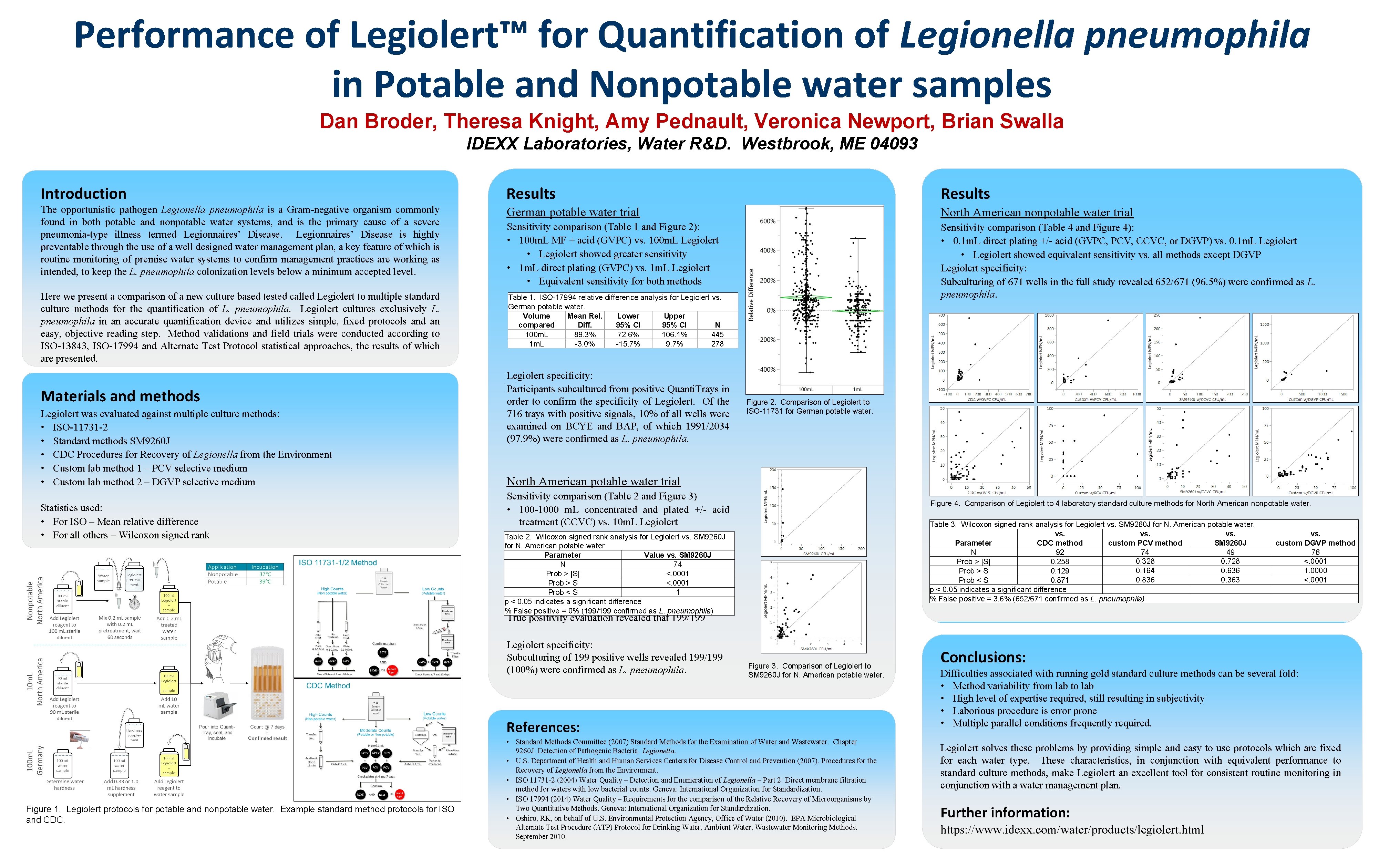
Performance of Legiolert™ for Quantification of Legionella pneumophila in Potable and Nonpotable water samples Dan Broder, Theresa Knight, Amy Pednault, Veronica Newport, Brian Swalla IDEXX Laboratories, Water R&D. Westbrook, ME 04093 Introduction Results The opportunistic pathogen Legionella pneumophila is a Gram-negative organism commonly found in both potable and nonpotable water systems, and is the primary cause of a severe pneumonia-type illness termed Legionnaires’ Disease is highly preventable through the use of a well designed water management plan, a key feature of which is routine monitoring of premise water systems to confirm management practices are working as intended, to keep the L. pneumophila colonization levels below a minimum accepted level. German potable water trial North American nonpotable water trial Sensitivity comparison (Table 1 and Figure 2): • 100 m. L MF + acid (GVPC) vs. 100 m. L Legiolert • Legiolert showed greater sensitivity • 1 m. L direct plating (GVPC) vs. 1 m. L Legiolert • Equivalent sensitivity for both methods Here we present a comparison of a new culture based tested called Legiolert to multiple standard culture methods for the quantification of L. pneumophila. Legiolert cultures exclusively L. pneumophila in an accurate quantification device and utilizes simple, fixed protocols and an easy, objective reading step. Method validations and field trials were conducted according to ISO-13843, ISO-17994 and Alternate Test Protocol statistical approaches, the results of which are presented. Table 1. ISO-17994 relative difference analysis for Legiolert vs. German potable water. Volume Mean Rel. Lower Upper compared Diff. 95% CI N 100 m. L 89. 3% 72. 6% 106. 1% 445 1 m. L -3. 0% -15. 7% 9. 7% 278 Sensitivity comparison (Table 4 and Figure 4): • 0. 1 m. L direct plating +/- acid (GVPC, PCV, CCVC, or DGVP) vs. 0. 1 m. L Legiolert • Legiolert showed equivalent sensitivity vs. all methods except DGVP Legiolert specificity: Subculturing of 671 wells in the full study revealed 652/671 (96. 5%) were confirmed as L. pneumophila. Materials and methods Legiolert was evaluated against multiple culture methods: • ISO-11731 -2 • Standard methods SM 9260 J • CDC Procedures for Recovery of Legionella from the Environment • Custom lab method 1 – PCV selective medium • Custom lab method 2 – DGVP selective medium Statistics used: • For ISO – Mean relative difference • For all others – Wilcoxon signed rank Legiolert specificity: Participants subcultured from positive Quanti. Trays in order to confirm the specificity of Legiolert. Of the 716 trays with positive signals, 10% of all wells were examined on BCYE and BAP, of which 1991/2034 (97. 9%) were confirmed as L. pneumophila. Figure 2. Comparison of Legiolert to ISO-11731 for German potable water. North American potable water trial Sensitivity comparison (Table 2 and Figure 3) • 100 -1000 m. L concentrated and plated +/- acid treatment (CCVC) vs. 10 m. L Legiolert • Wilcoxon Legiolert showed greaterforsensitivity Table 2. signed rank analysis Legiolert vs. SM 9260 J Figure 4. Comparison of Legiolert to 4 laboratory standard culture methods for North American nonpotable water. Table 3. Wilcoxon signed rank analysis for Legiolert vs. SM 9260 J for N. American potable water. vs. vs. Parameter CDC method custom PCV method SM 9260 J N 92 74 49 0. 328 0. 728 Prob > |S| 0. 258 0. 164 0. 636 Prob > S 0. 129 0. 836 0. 363 Prob < S 0. 871 p < 0. 05 indicates a significant difference % False positive = 3. 6% (652/671 confirmed as L. pneumophila) for N. American potable water Parameter Value vs. SM 9260 J N 74 Prob > |S| <. 0001 Prob > S <. 0001 Prob < S 1 p < 0. 05 indicates a significant difference % False positive = 0% (199/199 confirmed as L. pneumophila) vs. custom DGVP method 76 <. 0001 1. 0000 <. 0001 True positivity evaluation revealed that 199/199 Legiolert specificity: Subculturing of 199 positive wells revealed 199/199 (100%) were confirmed as L. pneumophila. Figure 3. Comparison of Legiolert to SM 9260 J for N. American potable water. References: Figure 1. Legiolert protocols for potable and nonpotable water. Example standard method protocols for ISO and CDC. • Standard Methods Committee (2007) Standard Methods for the Examination of Water and Wastewater. Chapter 9260 J: Detection of Pathogenic Bacteria. Legionella. • U. S. Department of Health and Human Services Centers for Disease Control and Prevention (2007). Procedures for the Recovery of Legionella from the Environment. • ISO 11731 -2 (2004) Water Quality – Detection and Enumeration of Legionella – Part 2: Direct membrane filtration method for waters with low bacterial counts. Geneva: International Organization for Standardization. • ISO 17994 (2014) Water Quality – Requirements for the comparison of the Relative Recovery of Microorganisms by Two Quantitative Methods. Geneva: International Organization for Standardization. • Oshiro, RK, on behalf of U. S. Environmental Protection Agency, Office of Water (2010). EPA Microbiological Alternate Test Procedure (ATP) Protocol for Drinking Water, Ambient Water, Wastewater Monitoring Methods. September 2010. Conclusions: Difficulties associated with running gold standard culture methods can be several fold: • Method variability from lab to lab • High level of expertise required, still resulting in subjectivity • Laborious procedure is error prone • Multiple parallel conditions frequently required. Legiolert solves these problems by providing simple and easy to use protocols which are fixed for each water type. These characteristics, in conjunction with equivalent performance to standard culture methods, make Legiolert an excellent tool for consistent routine monitoring in conjunction with a water management plan. Further information: https: //www. idexx. com/water/products/legiolert. html

