NC STATE UNIVERSITY Identification of AFLP markers linked
- Slides: 1

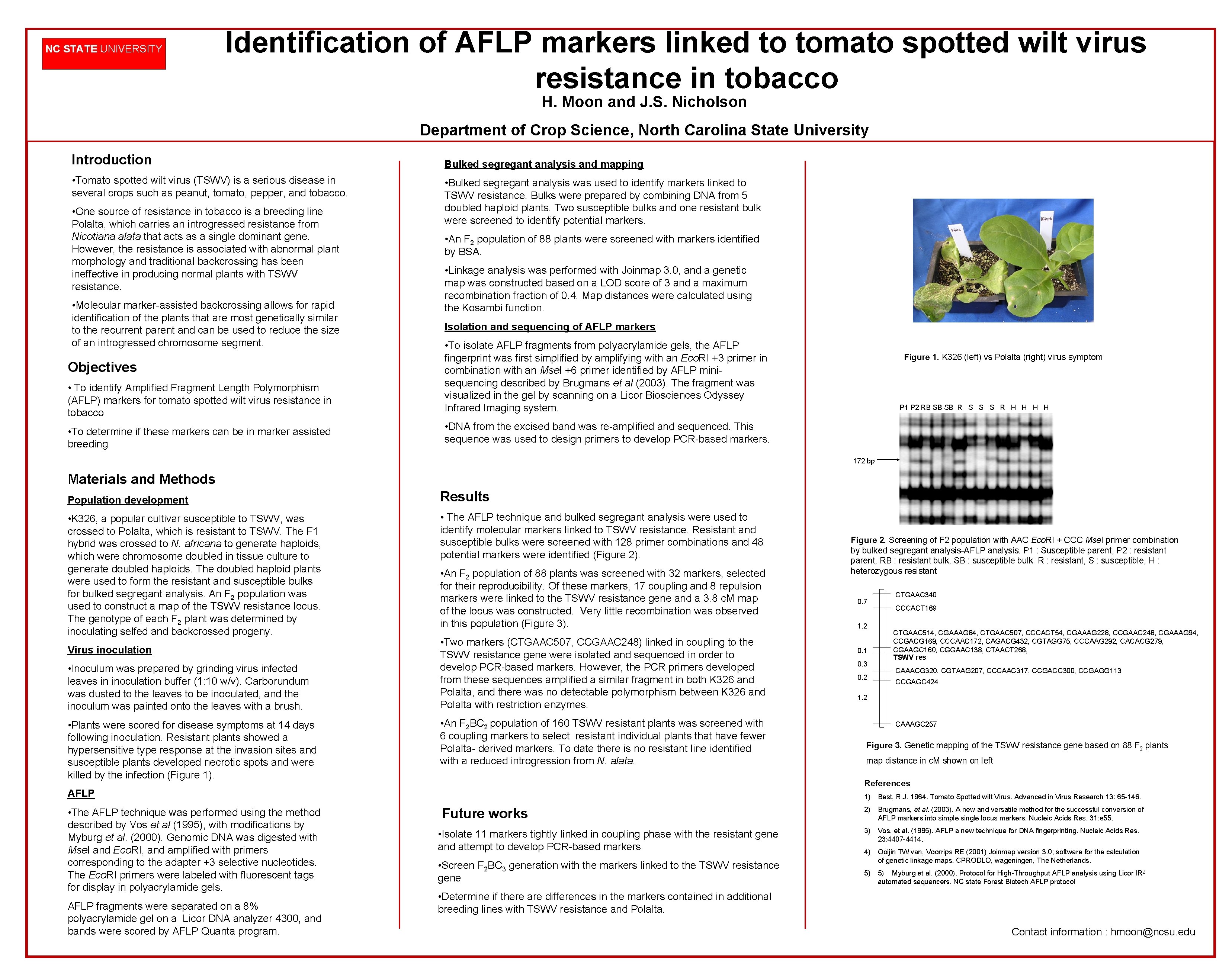
NC STATE UNIVERSITY Identification of AFLP markers linked to tomato spotted wilt virus resistance in tobacco H. Moon and J. S. Nicholson Department of Crop Science, North Carolina State University Introduction • Tomato spotted wilt virus (TSWV) is a serious disease in several crops such as peanut, tomato, pepper, and tobacco. • One source of resistance in tobacco is a breeding line Polalta, which carries an introgressed resistance from Nicotiana alata that acts as a single dominant gene. However, the resistance is associated with abnormal plant morphology and traditional backcrossing has been ineffective in producing normal plants with TSWV resistance. • Molecular marker-assisted backcrossing allows for rapid identification of the plants that are most genetically similar to the recurrent parent and can be used to reduce the size of an introgressed chromosome segment. Bulked segregant analysis and mapping • Bulked segregant analysis was used to identify markers linked to TSWV resistance. Bulks were prepared by combining DNA from 5 doubled haploid plants. Two susceptible bulks and one resistant bulk were screened to identify potential markers. • An F 2 population of 88 plants were screened with markers identified by BSA. • Linkage analysis was performed with Joinmap 3. 0, and a genetic map was constructed based on a LOD score of 3 and a maximum recombination fraction of 0. 4. Map distances were calculated using the Kosambi function. Isolation and sequencing of AFLP markers • To identify Amplified Fragment Length Polymorphism (AFLP) markers for tomato spotted wilt virus resistance in tobacco • To isolate AFLP fragments from polyacrylamide gels, the AFLP fingerprint was first simplified by amplifying with an Eco. RI +3 primer in combination with an Mse. I +6 primer identified by AFLP minisequencing described by Brugmans et al (2003). The fragment was visualized in the gel by scanning on a Licor Biosciences Odyssey Infrared Imaging system. • To determine if these markers can be in marker assisted breeding • DNA from the excised band was re-amplified and sequenced. This sequence was used to design primers to develop PCR-based markers. Objectives Figure 1. K 326 (left) vs Polalta (right) virus symptom P 1 P 2 RB SB SB R S S S R H H 172 bp Materials and Methods Population development • K 326, a popular cultivar susceptible to TSWV, was crossed to Polalta, which is resistant to TSWV. The F 1 hybrid was crossed to N. africana to generate haploids, which were chromosome doubled in tissue culture to generate doubled haploids. The doubled haploid plants were used to form the resistant and susceptible bulks for bulked segregant analysis. An F 2 population was used to construct a map of the TSWV resistance locus. The genotype of each F 2 plant was determined by inoculating selfed and backcrossed progeny. Virus inoculation • Inoculum was prepared by grinding virus infected leaves in inoculation buffer (1: 10 w/v). Carborundum was dusted to the leaves to be inoculated, and the inoculum was painted onto the leaves with a brush. • Plants were scored for disease symptoms at 14 days following inoculation. Resistant plants showed a hypersensitive type response at the invasion sites and susceptible plants developed necrotic spots and were killed by the infection (Figure 1). Results • The AFLP technique and bulked segregant analysis were used to identify molecular markers linked to TSWV resistance. Resistant and susceptible bulks were screened with 128 primer combinations and 48 potential markers were identified (Figure 2). • An F 2 population of 88 plants was screened with 32 markers, selected for their reproducibility. Of these markers, 17 coupling and 8 repulsion markers were linked to the TSWV resistance gene and a 3. 8 c. M map of the locus was constructed. Very little recombination was observed in this population (Figure 3). • Two markers (CTGAAC 507, CCGAAC 248) linked in coupling to the TSWV resistance gene were isolated and sequenced in order to develop PCR-based markers. However, the PCR primers developed from these sequences amplified a similar fragment in both K 326 and Polalta, and there was no detectable polymorphism between K 326 and Polalta with restriction enzymes. • An F 2 BC 2 population of 160 TSWV resistant plants was screened with 6 coupling markers to select resistant individual plants that have fewer Polalta- derived markers. To date there is no resistant line identified with a reduced introgression from N. alata. AFLP fragments were separated on a 8% polyacrylamide gel on a Licor DNA analyzer 4300, and bands were scored by AFLP Quanta program. 0. 7 1. 2 0. 1 0. 3 0. 2 CTGAAC 340 CCCACT 169 CTGAAC 514, CGAAAG 84, CTGAAC 507, CCCACT 54, CGAAAG 228, CCGAAC 248, CGAAAG 94, CCGACG 169, CCCAAC 172, CAGACG 432, CGTAGG 75, CCCAAG 292, CACACG 279, CGAAGC 160, CGGAAC 138, CTAACT 268, TSWV res CAAACG 320, CGTAAG 207, CCCAAC 317, CCGACC 300, CCGAGG 113 CCGAGC 424 1. 2 CAAAGC 257 Figure 3. Genetic mapping of the TSWV resistance gene based on 88 F 2 plants map distance in c. M shown on left References AFLP • The AFLP technique was performed using the method described by Vos et al (1995), with modifications by Myburg et al. (2000). Genomic DNA was digested with Mse. I and Eco. RI, and amplified with primers corresponding to the adapter +3 selective nucleotides. The Eco. RI primers were labeled with fluorescent tags for display in polyacrylamide gels. Figure 2. Screening of F 2 population with AAC Eco. RI + CCC Mse. I primer combination by bulked segregant analysis-AFLP analysis. P 1 : Susceptible parent, P 2 : resistant parent, RB : resistant bulk, SB : susceptible bulk R : resistant, S : susceptible, H : heterozygous resistant 1) Best, R. J. 1964. Tomato Spotted wilt Virus. Advanced in Virus Research 13: 65 -146. Future works • Isolate 11 markers tightly linked in coupling phase with the resistant gene and attempt to develop PCR-based markers • Screen F 2 BC 3 generation with the markers linked to the TSWV resistance gene 2) Brugmans, et al. (2003). A new and versatile method for the successful conversion of AFLP markers into simple single locus markers. Nucleic Acids Res. 31: e 55. 3) Vos, et al. (1995). AFLP a new technique for DNA fingerprinting. Nucleic Acids Res. 23: 4407 -4414. 4) Ooijin TW van, Voorrips RE (2001) Joinmap version 3. 0; software for the calculation of genetic linkage maps. CPRODLO, wageningen, The Netherlands. 5) 5) Myburg et al. (2000). Protocol for High-Throughput AFLP analysis using Licor IR 2 automated sequencers. NC state Forest Biotech AFLP protocol • Determine if there are differences in the markers contained in additional breeding lines with TSWV resistance and Polalta. Contact information : hmoon@ncsu. edu

