Measurement in Science 1 Customary Units vs Metric







- Slides: 18

Measurement in Science 1

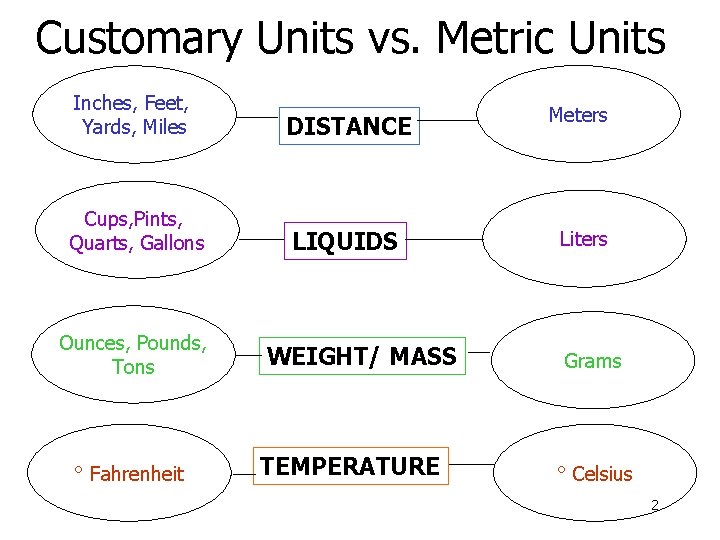
Customary Units vs. Metric Units Inches, Feet, Yards, Miles DISTANCE Meters Cups, Pints, Quarts, Gallons LIQUIDS Liters Ounces, Pounds, Tons ° Fahrenheit WEIGHT/ MASS TEMPERATURE Grams ° Celsius 2

What is a standard? It is an exact quantity that people agree to use for comparison. Why are measurement standards important? A meter in the U. S. is the same as a meter in France. 3

SI Units are. . . International Standards Abbreviated SI from the French Le Systeme Internationale d’Unites or the Common Name of the Metric System 4

Can you think about any situations that we use the Metric System in our everyday life – either home or at school? Name at least 3 examples: 1. Come on brain!! 2. 3. 5

Pros & Cons of Metric System Why should we use this system? • Pro’s – Easy: based on the use of ten – Conversions are easier - Just move decimal point – Used all over the world – not just USA • Con’s – History of conflict all over the world – People don’t want to change – Replacement cost of machinery too high 6

International Standard Units (SI) 7

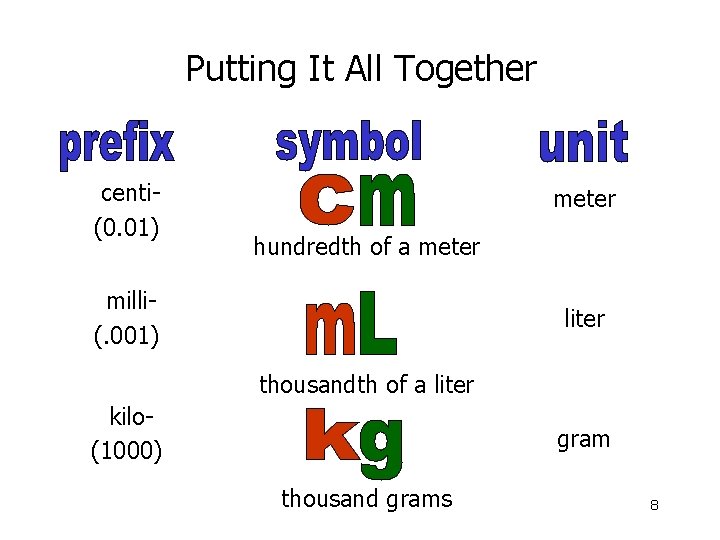
Putting It All Together centi(0. 01) meter hundredth of a meter milli(. 001) liter thousandth of a liter kilo(1000) gram thousand grams 8

From LARGEST to smallest the easiest way to remember the prefixes is in a sentence……. . King Henry dances merrily down center main. Or. . King Henry doesn’t milk dairy cows on Mondays. Kilo- Hecto- Deka- BASE deci- Meter, Liter, Grams centi- milli- 9

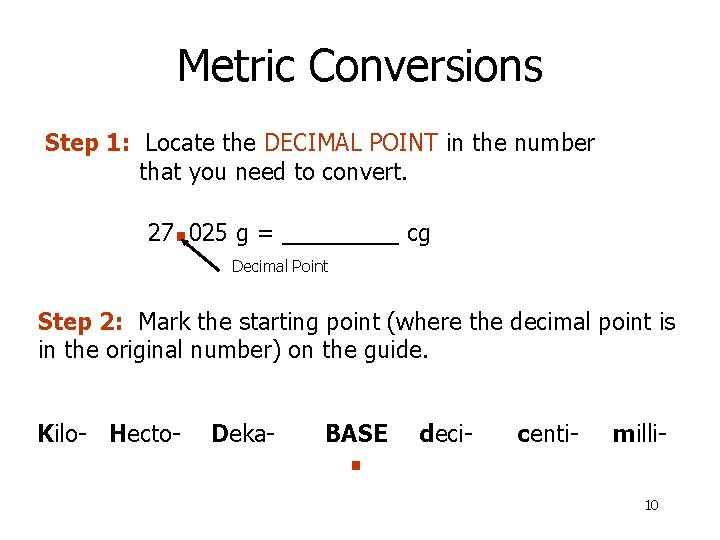
Metric Conversions Step 1: Locate the DECIMAL POINT in the number that you need to convert. . 27 025 g = _____ cg Decimal Point Step 2: Mark the starting point (where the decimal point is in the original number) on the guide. Kilo- Hecto- Deka- BASE . deci- centi- milli 10

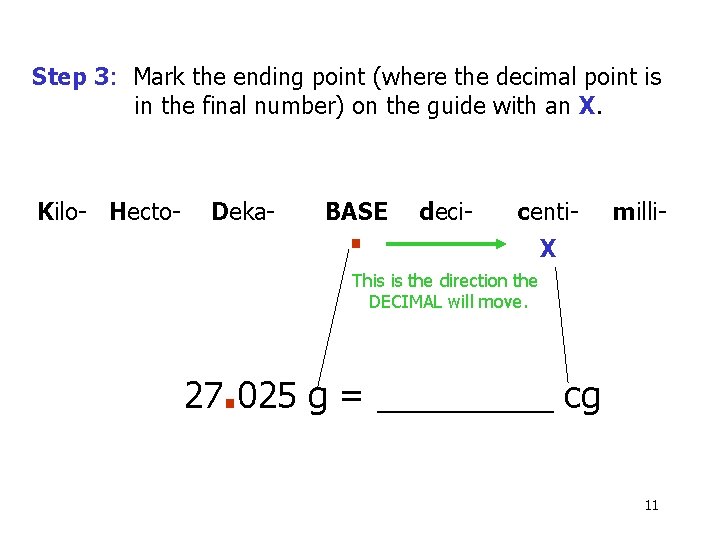
Step 3: Mark the ending point (where the decimal point is in the final number) on the guide with an X. Kilo- Hecto- Deka- BASE . deci- centi- milli- X This is the direction the DECIMAL will move. 27. 025 g = _____ cg 11

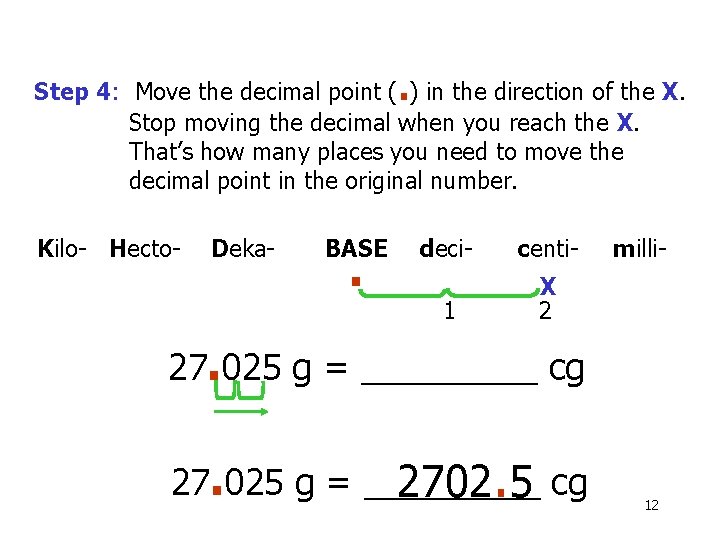
Step 4: Move the decimal point (. ) in the direction of the X. Stop moving the decimal when you reach the X. That’s how many places you need to move the decimal point in the original number. Kilo- Hecto- Deka- BASE . deci- centi- 1 X 2 milli- 27. 025 g = _____ cg 27. 025 g = _____ 2702. 5 cg 12

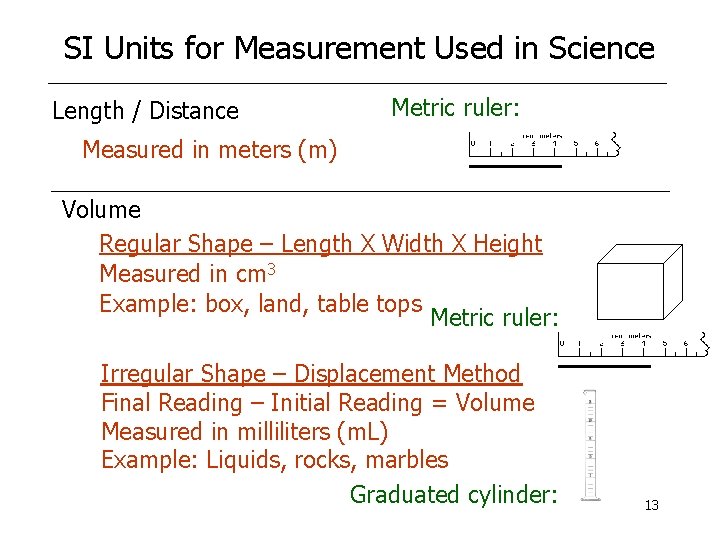
SI Units for Measurement Used in Science Length / Distance Metric ruler: Measured in meters (m) Volume Regular Shape – Length X Width X Height Measured in cm 3 Example: box, land, table tops Metric ruler: Irregular Shape – Displacement Method Final Reading – Initial Reading = Volume Measured in milliliters (m. L) Example: Liquids, rocks, marbles Graduated cylinder: 13

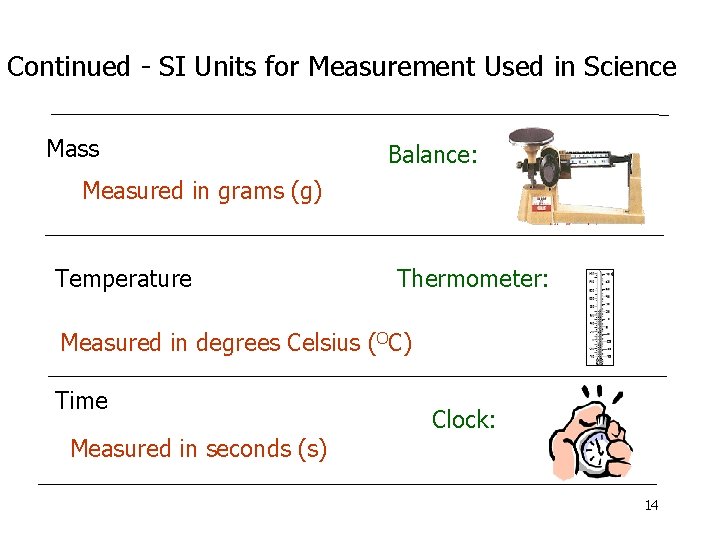
Continued - SI Units for Measurement Used in Science Mass Balance: Measured in grams (g) Temperature Thermometer: Measured in degrees Celsius (OC) Time Clock: Measured in seconds (s) 14

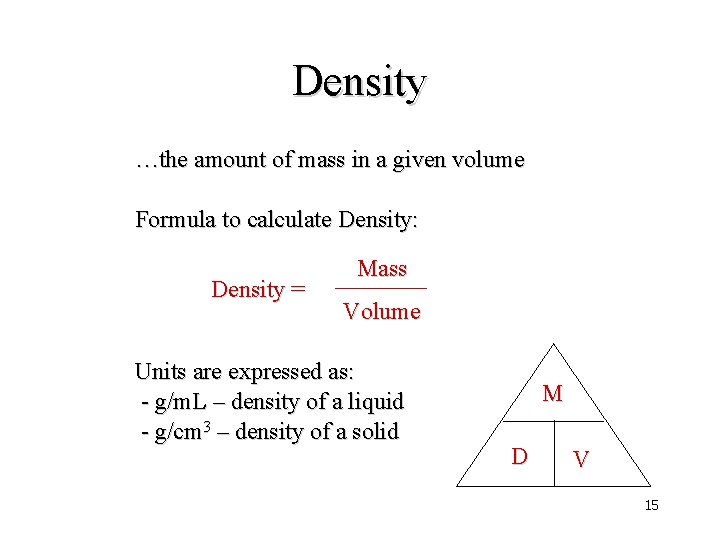
Density …the amount of mass in a given volume Formula to calculate Density: Density = Mass Volume Units are expressed as: - g/m. L – density of a liquid - g/cm 3 – density of a solid M D V 15

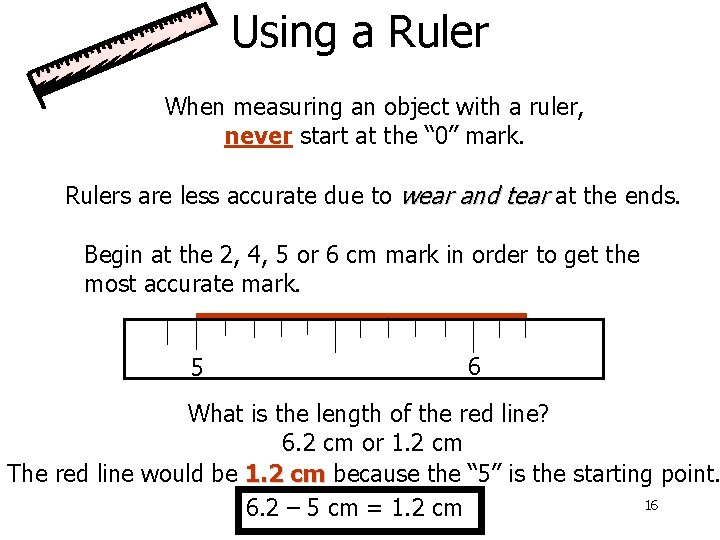
Using a Ruler When measuring an object with a ruler, never start at the “ 0” mark. Rulers are less accurate due to wear and tear at the ends. Begin at the 2, 4, 5 or 6 cm mark in order to get the most accurate mark. 5 6 What is the length of the red line? 6. 2 cm or 1. 2 cm The red line would be 1. 2 cm because the “ 5” is the starting point. 16 6. 2 – 5 cm = 1. 2 cm

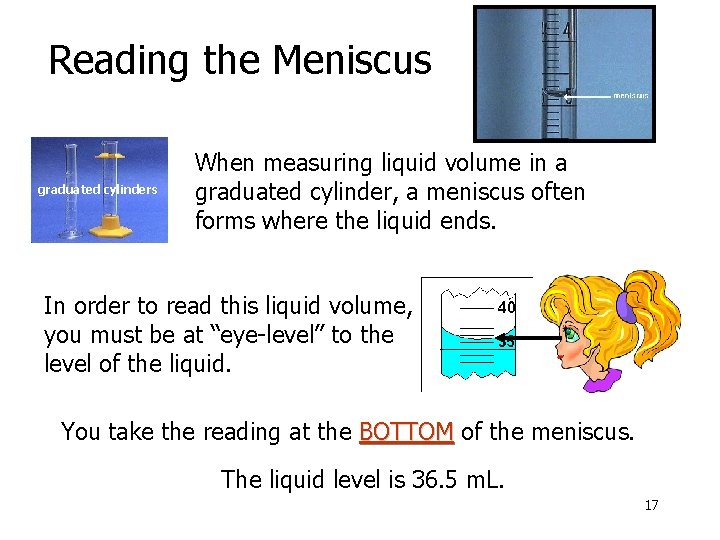
Reading the Meniscus graduated cylinders When measuring liquid volume in a graduated cylinder, a meniscus often forms where the liquid ends. In order to read this liquid volume, you must be at “eye-level” to the level of the liquid. You take the reading at the BOTTOM of the meniscus. The liquid level is 36. 5 m. L. 17

Read these liquid volumes in m. L: A. 76. 0 m. L B. 11. 5 m. L 18
 Customary and metric units
Customary and metric units Converting customary units
Converting customary units Customary units of measurement
Customary units of measurement Lesson 5 convert customary units of weight
Lesson 5 convert customary units of weight Customary units of measurement
Customary units of measurement Typical room height appropriate metric unit
Typical room height appropriate metric unit Pico unit
Pico unit Milli centi deci metre
Milli centi deci metre Which countries use imperial system
Which countries use imperial system Metric units
Metric units Brainpop metric vs customary answer key
Brainpop metric vs customary answer key Customary units of length
Customary units of length Customary units capacity
Customary units capacity Convert customary units of capacity
Convert customary units of capacity Graduated cylinder us customary units
Graduated cylinder us customary units Converting customary units of length
Converting customary units of length Metric mania conversion challenge
Metric mania conversion challenge Metric measurement virtual lab
Metric measurement virtual lab Metric system of measurement
Metric system of measurement