Last hour Selection rules for el dipole transitions

- Slides: 3

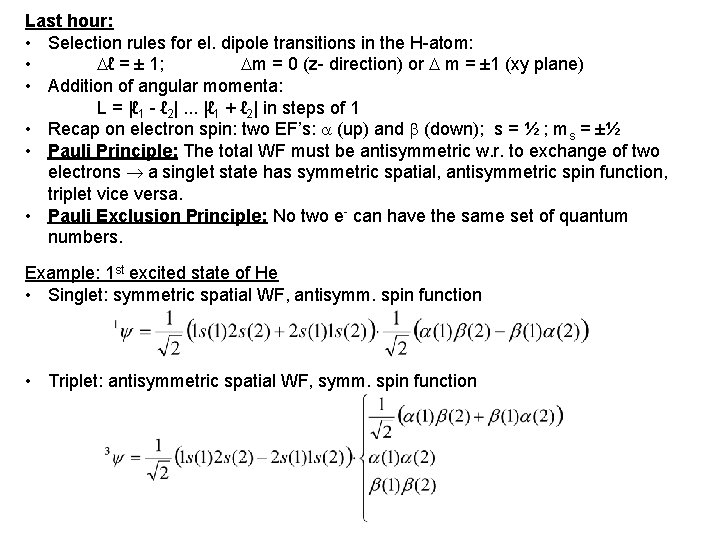
Last hour: • Selection rules for el. dipole transitions in the H-atom: • ℓ = ± 1; m = 0 (z- direction) or m = ± 1 (xy plane) • Addition of angular momenta: L = |ℓ 1 - ℓ 2|. . . |ℓ 1 + ℓ 2| in steps of 1 • Recap on electron spin: two EF’s: (up) and (down); s = ½ ; ms = ±½ • Pauli Principle: The total WF must be antisymmetric w. r. to exchange of two electrons a singlet state has symmetric spatial, antisymmetric spin function, triplet vice versa. • Pauli Exclusion Principle: No two e- can have the same set of quantum numbers. Example: 1 st excited state of He • Singlet: symmetric spatial WF, antisymm. spin function • Triplet: antisymmetric spatial WF, symm. spin function

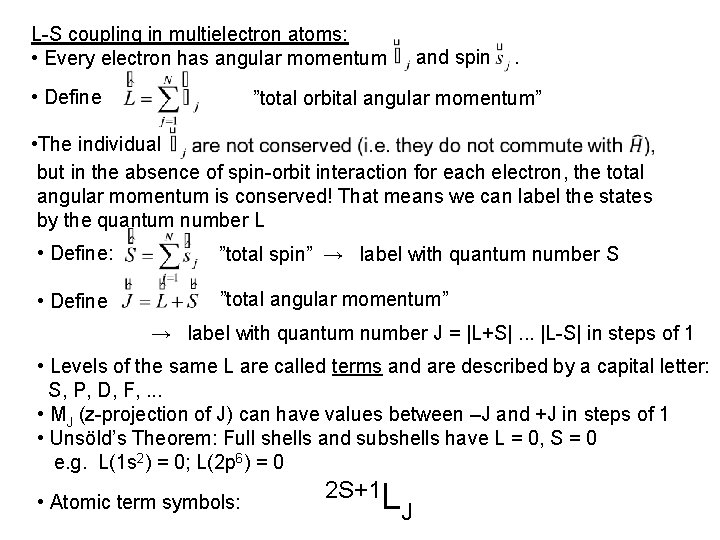
L-S coupling in multielectron atoms: • Every electron has angular momentum • Define and spin . ”total orbital angular momentum” • The individual but in the absence of spin-orbit interaction for each electron, the total angular momentum is conserved! That means we can label the states by the quantum number L • Define: ”total spin” → label with quantum number S • Define ”total angular momentum” → label with quantum number J = |L+S|. . . |L-S| in steps of 1 • Levels of the same L are called terms and are described by a capital letter: S, P, D, F, . . . • MJ (z-projection of J) can have values between –J and +J in steps of 1 • Unsöld’s Theorem: Full shells and subshells have L = 0, S = 0 e. g. L(1 s 2) = 0; L(2 p 6) = 0 • Atomic term symbols: 2 S+1 L J

John Robinson Pierce, American engineer and author. Coined the term "transistor”, wrote science fiction under the pseudonym “J. J. Coupling” http: //en. wikipedia. org/wiki/File: John_Robinson_Pierce. jpg