Improved Efficacy and Reduced Hospitalization Cost of TLIF




- Slides: 9

Improved Efficacy and Reduced Hospitalization Cost of TLIF Procedures Due to the Use of a Dedicated Device for Disc Space Preparation John Peloza, M. D. Michael Millgram, M. D. Erel Jacobian, B. Med. Sc Daniel Kolsky, M. Sc. Richard Guyer, M. D. Jean-Charles Le Huec, M. D. Ph. D. Ely Ashkenazi, M. D.

Disclosures • J. Peloza, E. Jacobian, D. Kolsky: None. • M. Millgram, R. Guyer, J. C. Le Huec: Consultants for Carevature Medical Ltd. • E. Ashkenazi: Consultant and shareholder for Carevature Medical Ltd.

Introduction • Transforaminal Lumbar Interbody Fusion (TLIF) procedures are commonly performed in the US, increasing in number as the population ages. • A new powered FDA-approved device can benefit this procedure’s outcome and reduce the time it requires.

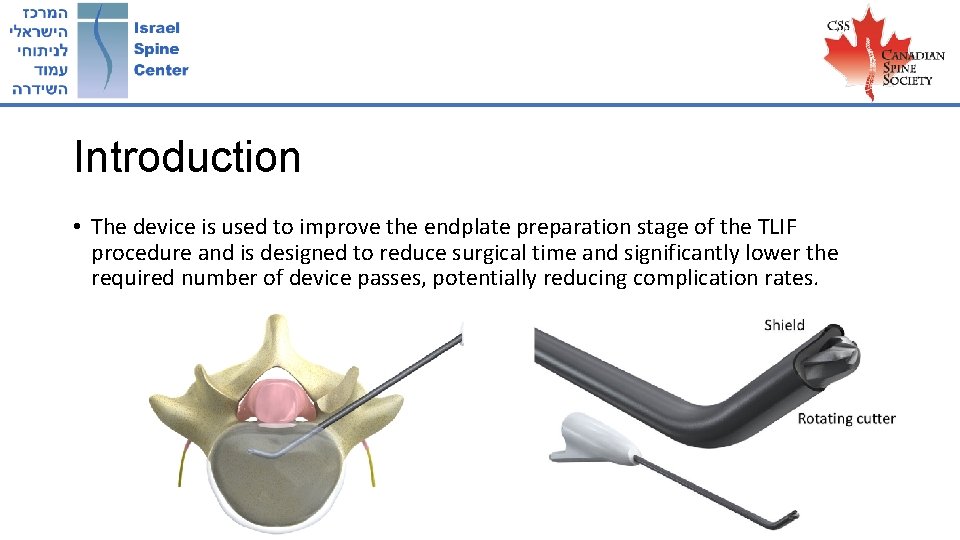
Introduction • The device is used to improve the endplate preparation stage of the TLIF procedure and is designed to reduce surgical time and significantly lower the required number of device passes, potentially reducing complication rates.

Objective • This study aims to assess the effect of device use on clinical outcome and hospitalization costs in a retrospective single center study.

Methods • The records of 208 single-level TLIF procedures conducted in a single hospital during 2012 -2019 were reviewed. • 143 conducted using the device • 65 controls • Surgery duration, length-of stay and complication rates were extracted from the records and compared between both groups. • The hospitalization cost changes were assessed using data from the financial department and the literature.

Results • 23 minutes reduction in OR time (after controlling for the procedure year and patient characteristics, p<0. 01) • Fewer patients in the device group (2. 8% vs. 9. 2%, p=0. 04) complained on post-operative pain or weakness in the leg, possibly due to the shorter surgery time and reduced need to retract the nerve root. • Device use was associated with a reduction of $2, 060 when compared with the control procedures (p<0. 01).

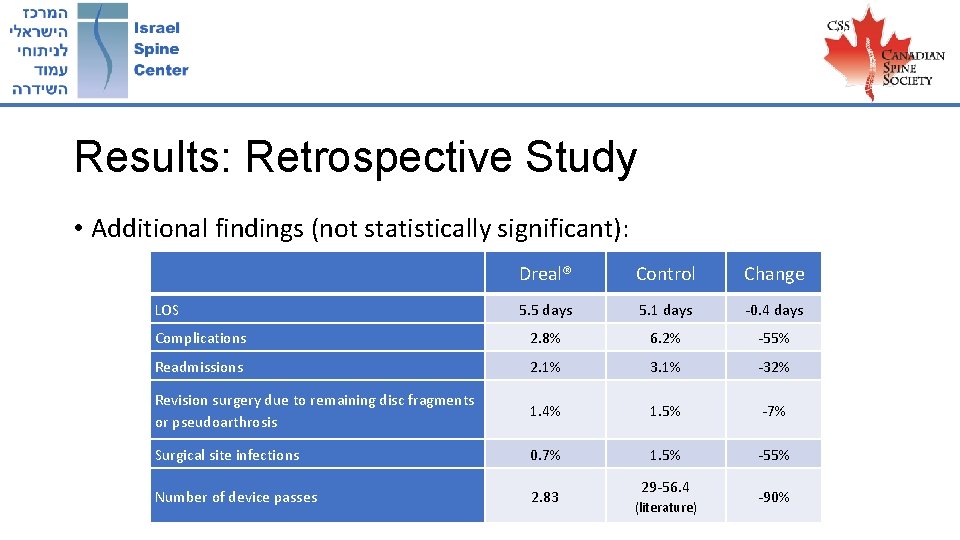
Results: Retrospective Study • Additional findings (not statistically significant): Dreal® Control Change 5. 5 days 5. 1 days -0. 4 days Complications 2. 8% 6. 2% -55% Readmissions 2. 1% 3. 1% -32% Revision surgery due to remaining disc fragments or pseudoarthrosis 1. 4% 1. 5% -7% Surgical site infections 0. 7% 1. 5% -55% Number of device passes 2. 83 LOS 29 -56. 4 (literature) -90%

Conclusions • The Dreal is Safe • Better and shorter endplate preparation with fewer instrument passes • Reduced complication rate • Lower hospitalization cost