Formation of a Copper Mirror A copper mirror


- Slides: 9

Formation of a Copper Mirror A copper mirror appears on the inside of a test tube when chemicals are mixed 3/7/2021 user@domain Jump to first page

Purpose n To show to make a copper mirror using hydrazine hydrate and copper (II) acetate. 3/7/2021 SDSMT user@domain Jump to first page

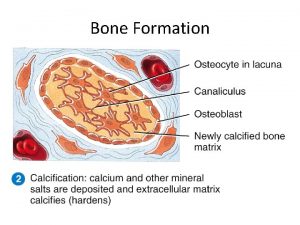
Demonstration n n 3/7/2021 A large test tube and a 250 m. L beaker to set the test tube in were the equipment used Copper (II) acetate was added to the test tube. Hydrazine hydrate was next added to the test tube. The test tube was rotated slowly while being heated gently over a micro-Bunsen burner. After the solution was heated for 1 -2 minutes copper metal plated out on the inside of the test tube. user@domain Jump to first page

Concepts n n n Organic Compounds Endothermic Reactions Redox Reactions 3/7/2021 user@domain Jump to first page

Inorganic Compounds n Inorganic Compound - Compound that is not carbon-based. n n The inorganic compound used in this experiment was hydrazine hydrate (molecular formula is H 2 NNH 2 · x H 2 O). Hydrazine hydrate is a reducing agent which means that it loses electron and is oxidized 3/7/2021 SDSMT user@domain Jump to first page

Endothermic Reactions n Endothermic Reaction - a reaction that absorbs heat from the surroundings. n In this demonstration heat was added to provide enough energy to cause the reaction to occur. A microburner was the heat source. 3/7/2021 SDSMT user@domain Jump to first page

Redox Reactions n Redox Reaction Oxidation-is the loss of electrons by a substance. Reduction-is the gain of electrons by a substance. • These happen simultaneously in a redox reaction. Ca + (s) + 2 H+ (aq) —› Ca 2+ (aq) + H 2 (g) n n Ca (s) is oxidized since it loses electrons and 2 H+ is reduced since it gains electrons. In this demonstration the following happens: Cu 2+ + 2 e- —› Cu n n 3/7/2021 The Cu 2+ is reduced since it gains electrons. The hydrazine hydrate gives up its electrons and becomes nitrogen gas, N 2 SDSMT user@domain Jump to first page

Conclusion n A copper mirror was made using hydrazine hydrate and copper (II) acetate 3/7/2021 SDSMT user@domain Jump to first page

Comments Hydrazine hydrate has also been used as a rocket fuel because of its ability to convert to nitrogen gas and provide thrust for rockets 3/7/2021 user@domain Jump to first page
 Copper mirror reaction
Copper mirror reaction A mirror with a flat surface
A mirror with a flat surface Mehran convex mirror
Mehran convex mirror Convex mirror is a diverging mirror
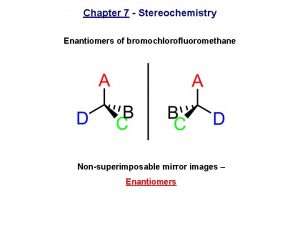
Convex mirror is a diverging mirror Bromochlorofluoromethane enantiomers
Bromochlorofluoromethane enantiomers Mirror mirror commonwealth fund
Mirror mirror commonwealth fund Formation of image of an extended object by a plane mirror
Formation of image of an extended object by a plane mirror Formation initiale vs formation continue
Formation initiale vs formation continue Empirical formula of copper chloride
Empirical formula of copper chloride Copper sun poem
Copper sun poem