FLIGHTFXR Study Tropifexor LJN 452 in NASH phase
- Slides: 4

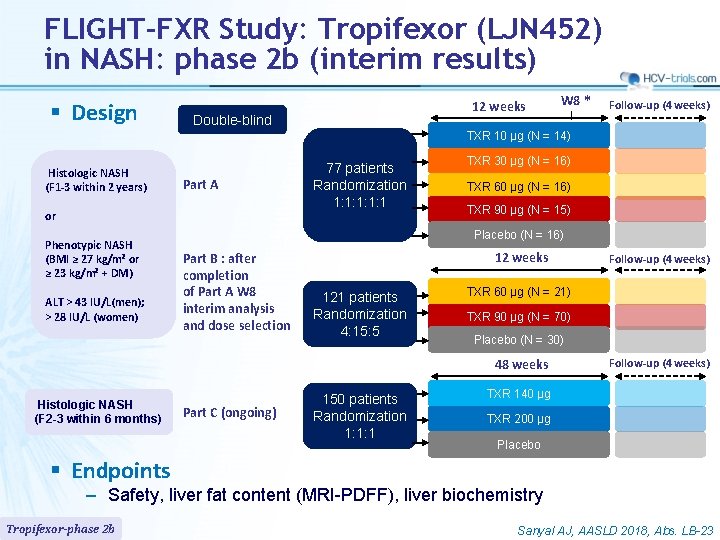
FLIGHT-FXR Study: Tropifexor (LJN 452) in NASH: phase 2 b (interim results) § Design 12 weeks Double-blind W 8 * Follow-up (4 weeks) TXR 10 µg (N = 14) Histologic NASH (F 1 -3 within 2 years) Part A or Phenotypic NASH (BMI ≥ 27 kg/m² or ≥ 23 kg/m² + DM) ALT > 43 IU/L(men); > 28 IU/L (women) 77 patients Randomization 1: 1: 1 TXR 30 µg (N = 16) TXR 60 µg (N = 16) TXR 90 µg (N = 15) Placebo (N = 16) Part B : after completion of Part A W 8 interim analysis and dose selection 12 weeks 121 patients Randomization 4: 15: 5 TXR 60 µg (N = 21) TXR 90 µg (N = 70) Placebo (N = 30) 48 weeks Histologic NASH (F 2 -3 within 6 months) Part C (ongoing) 150 patients Randomization 1: 1: 1 Follow-up (4 weeks) TXR 140 µg TXR 200 µg Placebo § Endpoints – Safety, liver fat content (MRI-PDFF), liver biochemistry Tropifexor-phase 2 b Sanyal AJ, AASLD 2018, Abs. LB-23

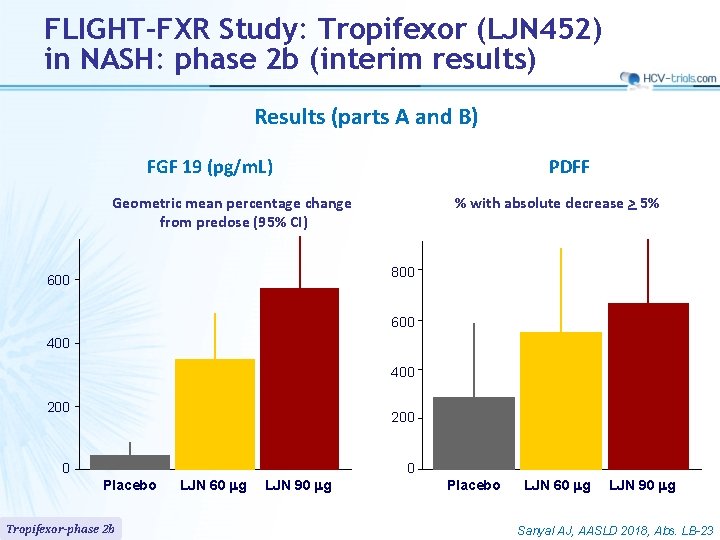
FLIGHT-FXR Study: Tropifexor (LJN 452) in NASH: phase 2 b (interim results) Results (parts A and B) FGF 19 (pg/m. L) PDFF Geometric mean percentage change from predose (95% CI) % with absolute decrease > 5% 800 600 400 200 0 0 Placebo Tropifexor-phase 2 b LJN 60 mg LJN 90 mg Placebo LJN 60 mg LJN 90 mg Sanyal AJ, AASLD 2018, Abs. LB-23

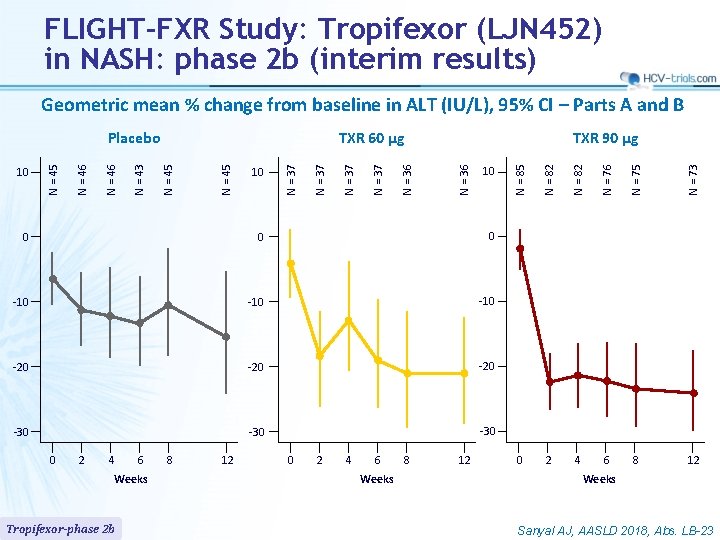
FLIGHT-FXR Study: Tropifexor (LJN 452) in NASH: phase 2 b (interim results) Geometric mean % change from baseline in ALT (IU/L), 95% CI – Parts A and B -10 -20 -20 -30 -30 0 2 4 6 Weeks Tropifexor-phase 2 b 8 12 0 2 4 6 Weeks 8 12 N = 73 -10 N = 75 0 N = 76 0 N = 82 10 N = 85 TXR 90 µg N = 36 N = 37 10 N = 37 N = 45 TXR 60 µg N = 45 N = 43 N = 46 10 N = 45 Placebo 0 2 4 6 8 12 Weeks Sanyal AJ, AASLD 2018, Abs. LB-23

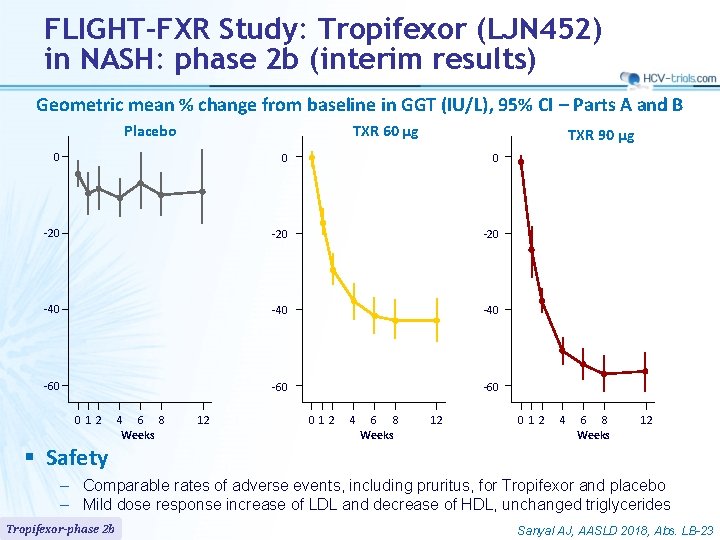
FLIGHT-FXR Study: Tropifexor (LJN 452) in NASH: phase 2 b (interim results) Geometric mean % change from baseline in GGT (IU/L), 95% CI – Parts A and B Placebo TXR 60 µg TXR 90 µg 0 0 0 -20 -20 -40 -40 -60 -60 012 § Safety 4 6 8 Weeks 12 012 4 6 8 Weeks 12 – Comparable rates of adverse events, including pruritus, for Tropifexor and placebo – Mild dose response increase of LDL and decrease of HDL, unchanged triglycerides Tropifexor-phase 2 b Sanyal AJ, AASLD 2018, Abs. LB-23