Equipoise and the ethics of clinical research When


![Two special cases l patients refractory to standard treatment – [I]t is more important Two special cases l patients refractory to standard treatment – [I]t is more important](https://slidetodoc.com/presentation_image_h2/0baf57e252cc6bb0fa38844f020b1423/image-3.jpg)






- Slides: 10

Equipoise and the ethics of clinical research When is it ethical to initiate a randomizedcontrolled trial? Freedman, NEJM, 1987. l “There exists (or in the case of novel therapy, there may soon exist) an honest, professional disagreement among expert clinicians about the preferred treatment. A clinical trial is instituted with the aim of resolving this dispute. ” l

Placebo-controlled trials and the logic of clinical purpose l When may the control be a placebo? Freedman, IRB, 1990. – no standard therapy – standard therapy no better than placebo – standard treatment is placebo – doubt regarding the net therapeutic advantage of standard therapy – standard treatment is unavailable (cost, supply).
![Two special cases l patients refractory to standard treatment It is more important Two special cases l patients refractory to standard treatment – [I]t is more important](https://slidetodoc.com/presentation_image_h2/0baf57e252cc6bb0fa38844f020b1423/image-3.jpg)
Two special cases l patients refractory to standard treatment – [I]t is more important to know ‘whether the treatment is better than nothing’ and will therefore offer an alternative for patients who do not have a response to the conventional treatment or cannot tolerate its adverse effects. ” (Solomon, letter, NEJM, 1995)

Two special cases l add-on treatments to standard therapy – “Patients are randomly assigned to receive a new drug or placebo, which is added to the existing treatment. Thus, patients in both the placebo and active treatment groups receive all medications that would normally be prescribed. ” (Gilbert, letter, NEJM, 1995)

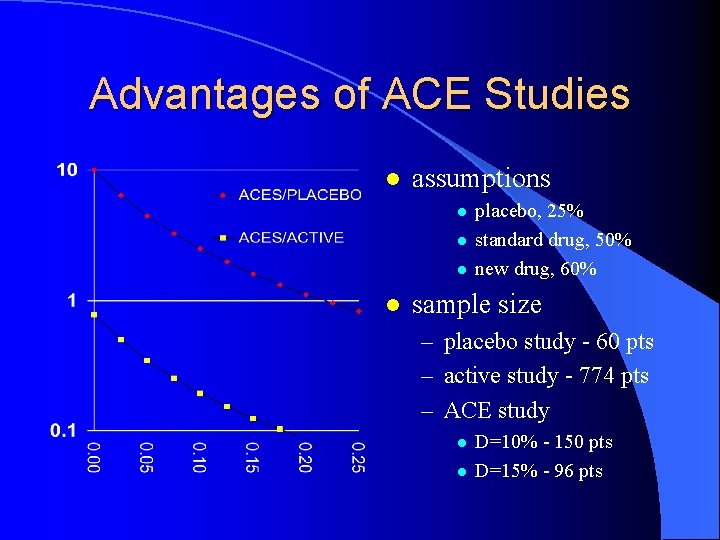
Advantages of ACE Studies l assumptions l l placebo, 25% standard drug, 50% new drug, 60% sample size – placebo study - 60 pts – active study - 774 pts – ACE study l l D=10% - 150 pts D=15% - 96 pts

Advantages of ACE study scientific and clinical advantages l more clinically relevant than placebocontrolled trial l A placebo-controlled trial asks: “Is this treatment better than nothing? ” l ACE study asks: “Is this treatment as good as what we are using now? ” l

Advantages of ACE study scientific and clinical advantages l possibility of using multiple hypotheses l – toxicity l a new treatment is of interest if it is roughly equally efficacious and has less side effects – negative symptoms l a new treatment is of interest if it is roughly equally efficacious and is better at treating negative symptoms

Advantages of ACE study regulatory advantages l a new drug may be superior to placebo but substantially inferior to standard treatment l Cost to society: l – “Many new drugs are expensive, and in some countries drug budgets are growing faster than other health care sectors…The key questions are: how much better are the new drugs than the old ones, how much more does it cost to obtain the additional benefits, and does the extra cost represent value for the money. (Henry and Hill, BMJ, 1995)

Advantages of ACE study ethical and legal advantages l patients are not knowingly given inferior treatment l possible liability: l – doctors owe a duty of care to their patients – an investigators chief concern ought to be the health and well being of his patient – providing a placebo may be negligent

Conclusion Placebo-controlled trials: Are they ethical? Are they necessary? l placebo controls may be acceptable in carefully defined circumstances (add-on treatment, treatment-resistant patients…) l ACE study is to be preferred: scientific, clinical, regulatory, ethical and legal advantages. l