Clinical equipoise the RCT dilemma Gopal Sreenivasan Crown























- Slides: 24

Clinical equipoise & the RCT dilemma Gopal Sreenivasan Crown Professor of Ethics Duke University

summary i. iii. problem: RCT permissible? standard solution: equipoise example

clinical trials • • is it permissible to do medical research with human beings? isn’t medical research basically just better, fancier clinical care? – no, they are fundamentally different

research vs. clinical care • they have fundamentally different aims – – medical research: to produce generalisable medical knowledge clinical care: to secure the best (health) interests of the patient

research vs. clinical care • their different aims appear to be in conflict, i. e. – – aims of clinical research appear inconsistent with the best health interests of the individual moreover, this is inherent in the nature of medical research

apparent conflict best seen as between investigator’s scientific duty to produce valid general knowledge investigator’s clinical duty to advance best health interests of individual subject

apparent conflict arises because RCT enrolment seems inconsistent with subject’s best health interests because of presumed inferiority of unproven experimental arm which ‘half’ the subjects will get

how to resolve? • which obligation should prevail in this conflict? – isn’t it obviously the obligation of the person as physician? • are not the interests of the individual sacrosanct?

how to resolve? • should not the obligation of the person as physician prevail? – – if the conflict is inherent in the nature of medical research, this entails not doing any research but medical practice depends on (past) research

conundrum? • indeed, the physician’s own obligation later – • to provide the best care to patients (in the future) depends on research now – conflict is between present and future patients

equipoise • appearance of conflict depends on the inference – – • not known not to be effective therefore, not effective but perhaps this is a mistake

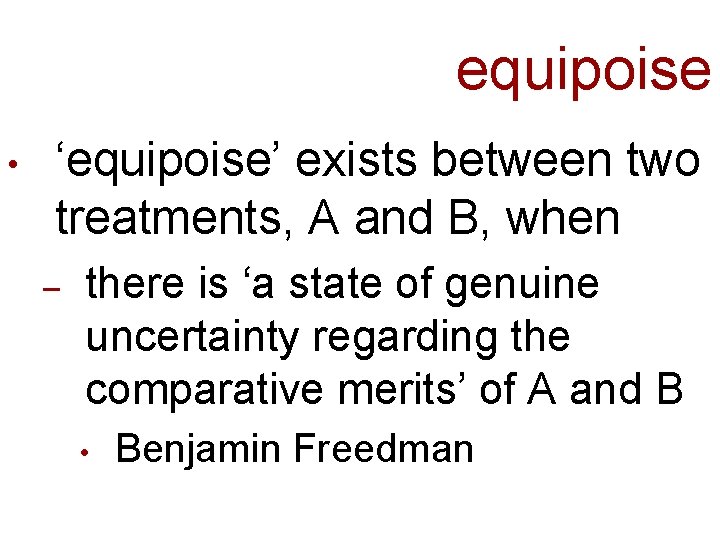
equipoise • ‘equipoise’ exists between two treatments, A and B, when – there is ‘a state of genuine uncertainty regarding the comparative merits’ of A and B • Benjamin Freedman

equipoise • if the investigator is in a state of equipoise between the experimental arm and the control arm of an RCT – – then she does not violate her obligation as physician for she does not knowingly offer inferior treatment to the subject

clinical equipoise requires existence of honest professional disagreement in expert community about which of trial’s two arms is clinically superior [assuming that ] one ≈ standard of care

solution RCT permissible when • i. ii. there is clinical equipoise regarding the two arms at the beginning of the trial is designed to make it reasonable to expect that successful results will disturb this equipoise

solution if investigator has a preference, why does this not violate her obligation as physician? • because the standards of professional responsibility are social in nature – • Freedman’s official answer

solution • but plausibility of this also implicitly relies on fact that medical community has a high(er) standard of evidence – indeed, a ‘gold’ standard: RCT! • that is why clinical equipoise is robust: disturbed only by RCT

example • consider 2003 study of letrozole after tamoxifen for breast cancer – result announced in PE Goss et alia, NEJM (2003) 349: 17931802.

2003 example • news items concerned fact that the study was interrupted mid-course – preliminary analysis confirmed significant benefit from experimental intervention

yay! • • to wit, a 43 percent reduction in risk of a recurrence or of new contralateral breast cancer study was stopped and placebo group was allowed to cross-over

but • study was stopped – • after 2. 4 years primary aim of the study was therefore not realised – to study 5 year effect

oh • therefore cannot document – a ‘survival’ advantage • – defined in terms of 5 years recommendation for 5 (or indeed > 2. 4) year treatment • data do not support

uh oh? • stopping decision ‘undeniably diminishes the clinical usefulness of the data’ – Bryant and Wolmark • accompanying NEJM editorial

thank you
 Clinical equipoise
Clinical equipoise Clinical equipoise
Clinical equipoise Vinodh gopal
Vinodh gopal Shuba gopal
Shuba gopal Gopal kakivaya
Gopal kakivaya Gopal vijayaraghavan
Gopal vijayaraghavan Gopal kutwaroo
Gopal kutwaroo Rct
Rct 3 types of bias
3 types of bias Rct brisbane
Rct brisbane Rct-822
Rct-822 Loi du 16 décembre 2010
Loi du 16 décembre 2010 Root canal obturation materials
Root canal obturation materials Rct children's services
Rct children's services Rct-274
Rct-274 Biasnn
Biasnn Bias rct
Bias rct Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Chụp tư thế worms-breton
Chụp tư thế worms-breton Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Ví dụ về giọng cùng tên
Ví dụ về giọng cùng tên Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới