Engr D 2190 Lecture 13 Concept Process Analysis



- Slides: 16

Engr. D 2190 – Lecture 13 Concept: Process Analysis by Mathematical Modeling – Energy Balances Context: Processes with Chemical Reactions – Heat of Reaction and Adiabatic Temperature Change Defining Question: What are the energy criteria for a viable chemical process? Read Chapter 3 pp. 139 -145 (energy balances, cont’d) Lecture 14 will follow the textbook. Bring your textbook and annotate.

Prelim 1: Tuesday 9/28, 7: 30 -9: 30 p. m. 245 Olin Hall Covers Chapter 2 and mass balances (formal and informal). Covers through Lecture 10, Homework 3, Calculation Session 4. Open book, open notes, open exercise solutions. Bring a calculator. Graphing calculators are allowed. Laptops only for digital textbook and material stored on laptop. Must be approved pre-prelim. Practice Exercises for Prelim 1. Optional - do not submit solutions. Process Design with real chemicals: 2. 18 Process Design with qualitative, informal mass balances: 3. 123 and 3. 132 Formal Mass Balances: 3. 20, 3. 25, and 3. 45 Informal Mass Balances: 3. 41 Solutions are posted.


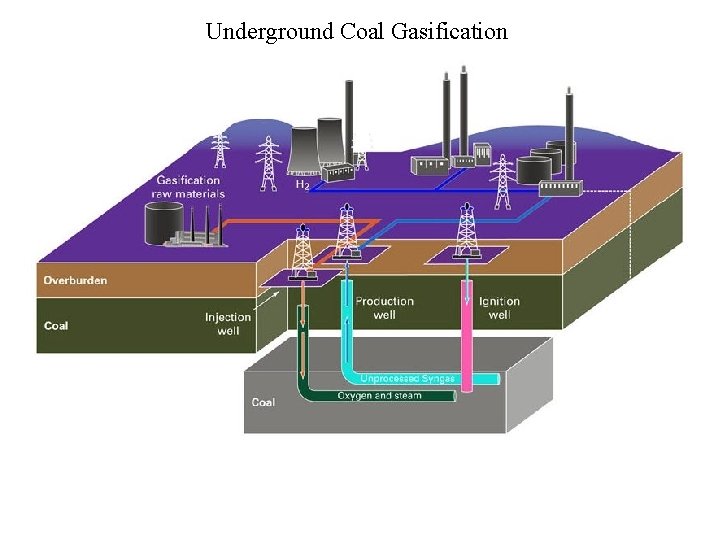
Underground Coal Gasification

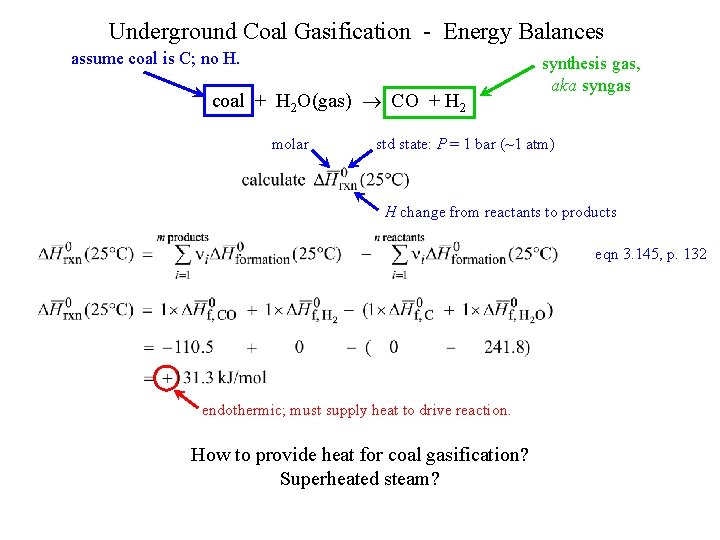
Underground Coal Gasification - Energy Balances assume coal is C; no H. coal + H 2 O(gas) CO + H 2 molar synthesis gas, aka syngas std state: P = 1 bar (~1 atm) H change from reactants to products eqn 3. 145, p. 132 endothermic; must supply heat to drive reaction. How to provide heat for coal gasification? Superheated steam?

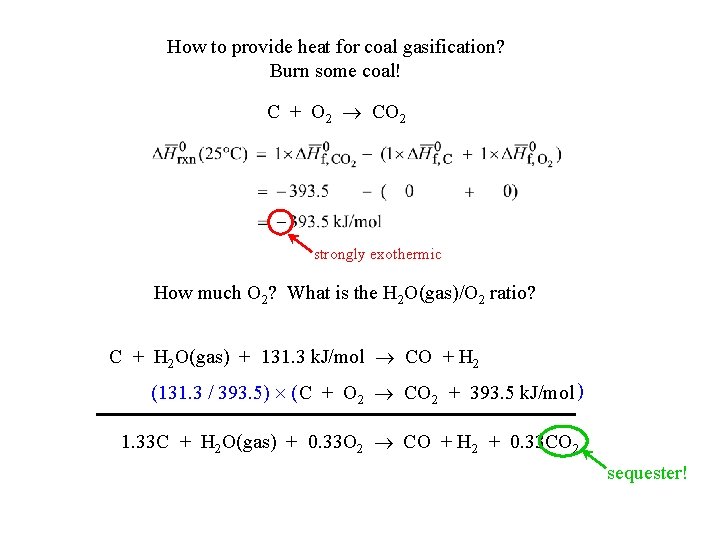
How to provide heat for coal gasification? Burn some coal! C + O 2 CO 2 strongly exothermic How much O 2? What is the H 2 O(gas)/O 2 ratio? C + H 2 O(gas) + 131. 3 k. J/mol CO + H 2 (131. 3 / 393. 5) ( C + O 2 CO 2 + 393. 5 k. J/mol ) 1. 33 C + H 2 O(gas) + 0. 33 O 2 CO + H 2 + 0. 33 CO 2 sequester!

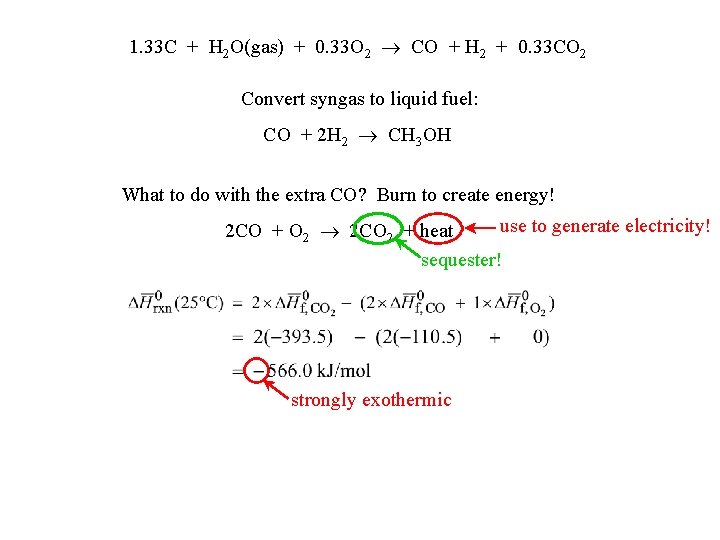
1. 33 C + H 2 O(gas) + 0. 33 O 2 CO + H 2 + 0. 33 CO 2 Convert syngas to liquid fuel: CO + 2 H 2 CH 3 OH What to do with the extra CO? Burn to create energy! use to generate electricity! 2 CO + O 2 2 CO 2 + heat sequester! strongly exothermic

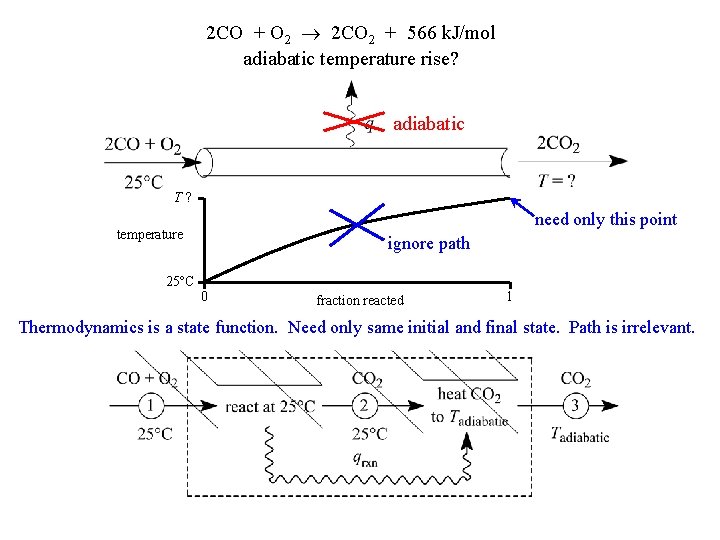
2 CO + O 2 2 CO 2 + 566 k. J/mol adiabatic temperature rise? adiabatic T? need only this point temperature ignore path 25 C 0 fraction reacted 1 Thermodynamics is a state function. Need only same initial and final state. Path is irrelevant.

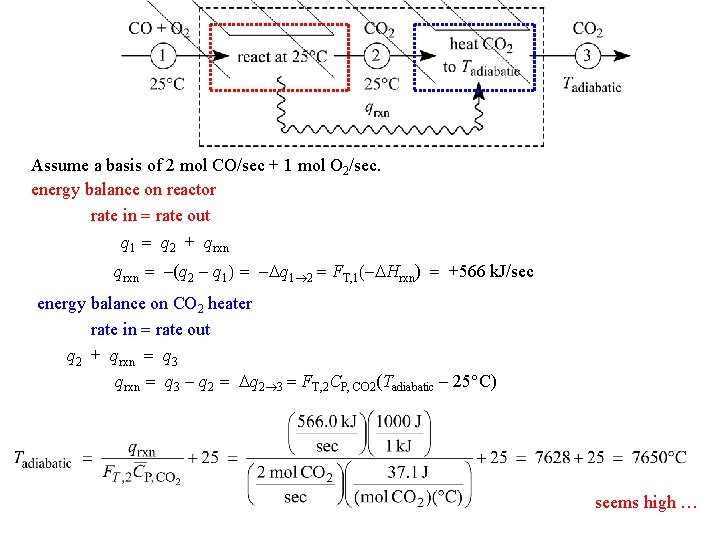
Assume a basis of 2 mol CO/sec + 1 mol O 2/sec. energy balance on reactor rate in = rate out q 1 = q 2 + qrxn = -(q 2 - q 1) = -Dq 1 2 = FT, 1(-DHrxn) = +566 k. J/sec energy balance on CO 2 heater rate in = rate out q 2 + qrxn = q 3 - q 2 = Dq 2 3 = FT, 2 CP, CO 2(Tadiabatic - 25 C) seems high …

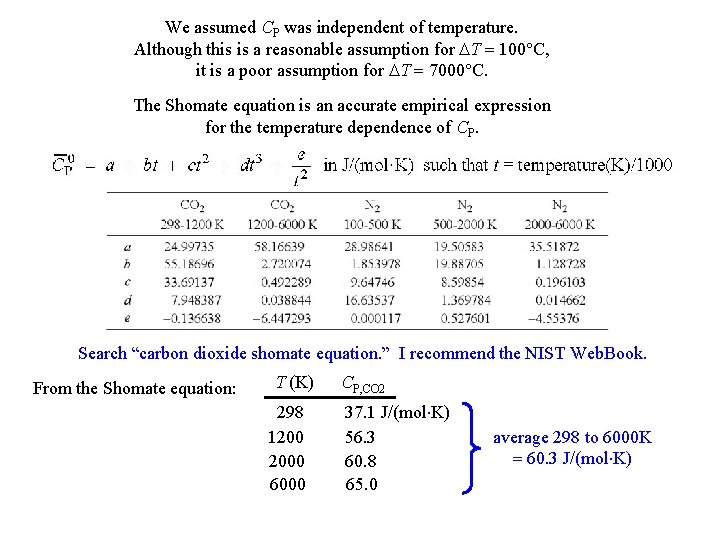
We assumed CP was independent of temperature. Although this is a reasonable assumption for DT = 100°C, it is a poor assumption for DT = 7000°C. The Shomate equation is an accurate empirical expression for the temperature dependence of CP. Search “carbon dioxide shomate equation. ” I recommend the NIST Web. Book. From the Shomate equation: T (K) 298 1200 2000 6000 CP, CO 2 37. 1 J/(mol K) 56. 3 60. 8 65. 0 average 298 to 6000 K = 60. 3 J/(mol K)

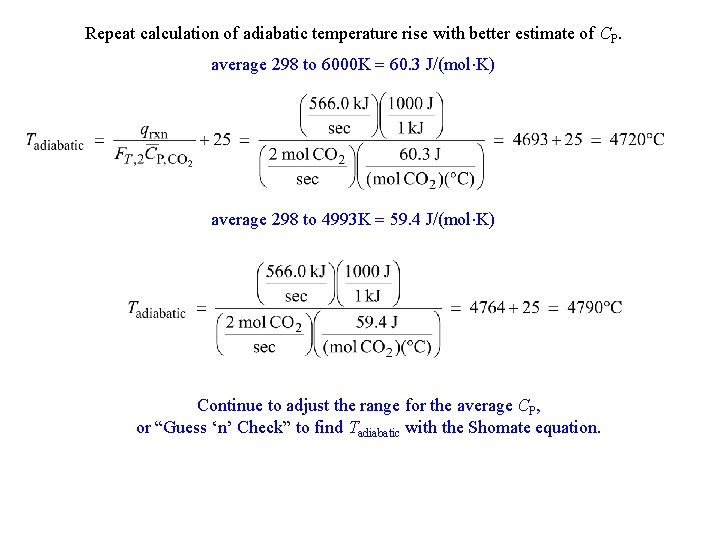
Repeat calculation of adiabatic temperature rise with better estimate of CP. average 298 to 6000 K = 60. 3 J/(mol K) average 298 to 4993 K = 59. 4 J/(mol K) Continue to adjust the range for the average CP, or “Guess ‘n’ Check” to find Tadiabatic with the Shomate equation.

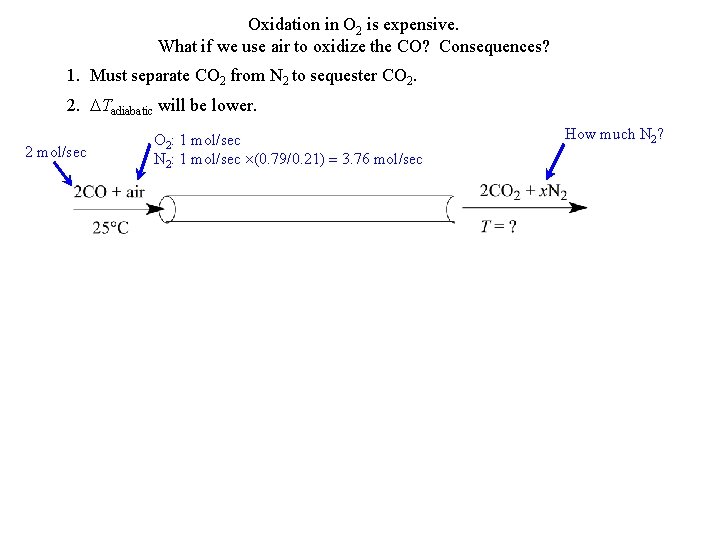
Oxidation in O 2 is expensive. What if we use air to oxidize the CO? Consequences? 1. Must separate CO 2 from N 2 to sequester CO 2. 2. DTadiabatic will be lower. 2 mol/sec O 2: 1 mol/sec N 2: 1 mol/sec (0. 79/0. 21) = 3. 76 mol/sec How much N 2?

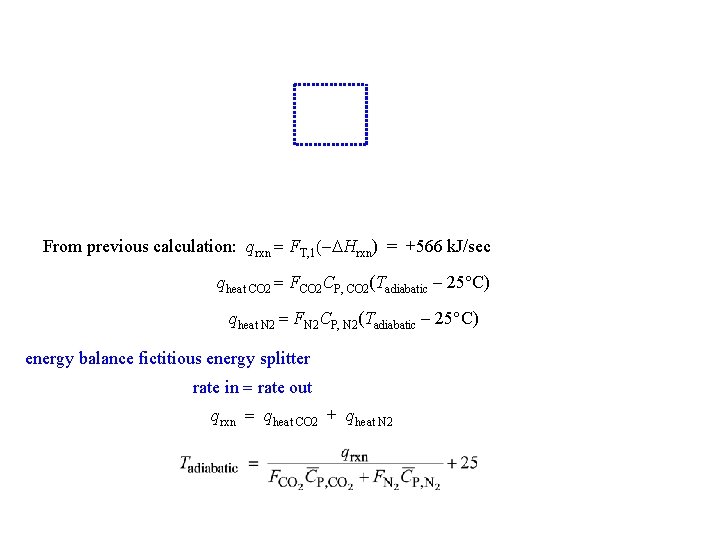
From previous calculation: qrxn = FT, 1(-DHrxn) = +566 k. J/sec qheat CO 2 = FCO 2 CP, CO 2(Tadiabatic - 25 C) qheat N 2 = FN 2 CP, N 2(Tadiabatic - 25 C) energy balance fictitious energy splitter rate in = rate out qrxn = qheat CO 2 + qheat N 2

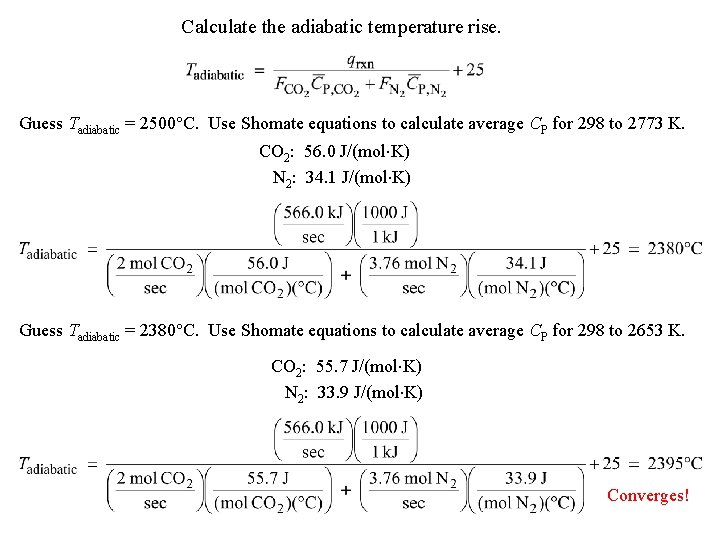
Calculate the adiabatic temperature rise. Guess Tadiabatic = 2500°C. Use Shomate equations to calculate average CP for 298 to 2773 K. CO 2: 56. 0 J/(mol K) N 2: 34. 1 J/(mol K) Guess Tadiabatic = 2380°C. Use Shomate equations to calculate average CP for 298 to 2653 K. CO 2: 55. 7 J/(mol K) N 2: 33. 9 J/(mol K) Converges!

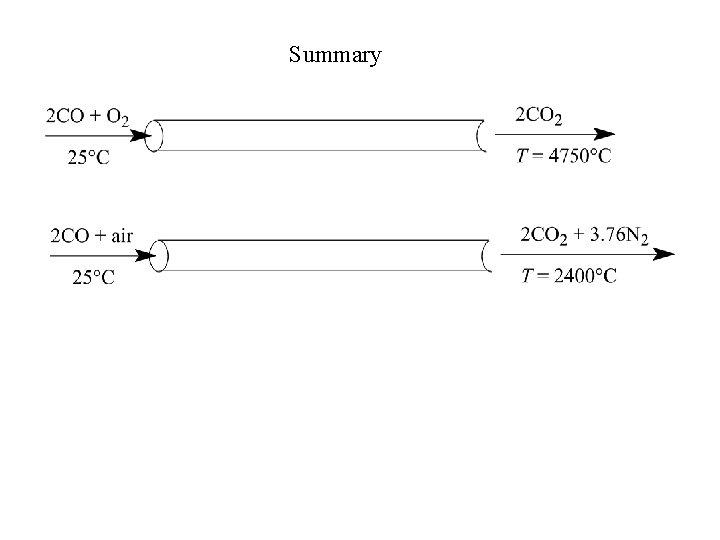
Summary

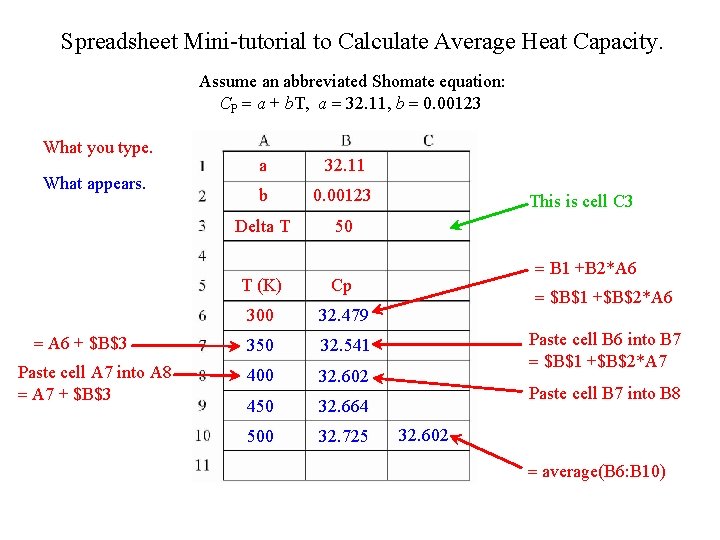
Spreadsheet Mini-tutorial to Calculate Average Heat Capacity. Assume an abbreviated Shomate equation: CP = a + b. T, a = 32. 11, b = 0. 00123 What you type. What appears. = A 6 + $B$3 Paste cell A 7 into A 8 = A 7 + $B$3 a 32. 11 b 0. 00123 Delta T 50 T (K) Cp 300 32. 479 350 32. 541 400 32. 602 450 32. 664 500 32. 725 This is cell C 3 = B 1 +B 2*A 6 = $B$1 +$B$2*A 6 Paste cell B 6 into B 7 = $B$1 +$B$2*A 7 Paste cell B 7 into B 8 32. 602 = average(B 6: B 10)