Choosing Polio Vaccines after Eradication of Polio Is






- Slides: 13

Choosing Polio Vaccines after Eradication of Polio: Is the Cost of A Vaccine Switch Prohibitively High? Prepared by Xingzhu Liu Ann Levin Marty Makinen Date: May 2, 2002 The PHRplus Project is funded by U. S. Agency for International Development and implemented by: Abt Associates Inc. and partners, Development Associates, Inc. ; Emory University Rollins School of Public Health; Philoxenia International Travel, Inc. Program for Appropriate Technology in Health; SAG Corp. ; Social Sectors Development Strategies, Inc. ; Training Resources Group; Tulane University School of Public Health and Tropical Medicine; University Research Co. , LLC.

I. Introduction 1. Decisions to be made w w w The world will be certified polio free in 2005 Should vaccinations be terminated or continued? If continued, what vaccine should be used (OPV vs IPV)? 2. If the choice is OPV w w w Occurrence of VAPP (600 per year) Transmission of vaccine virus Risk of vaccine virus reverts

I. Introduction (Cont’d) 3. If the choice is IPV w w w Risk of vaccine virus reverts The cost is high The risk of outbreak may increase w w w Low coverage rate Wild virus escape Transmission of reverted OPV virus 4. Objective w To estimate the incremental cost of the vaccine switch from OPV to IPV in developing countries

II. Methods 1. Target countries ---- All developing countries w Low coverage countries: <=50% w Intermediate coverage countries: 51 -79% w High coverage countries: >=80% 2. Definition of incremental cost w Net increase in total annual cost of polio immunization due to a vaccine switch from OPV to IPV 3. Selection of relevant costs w Exhaustive list of the types of cost w Identification of costs that may change w Costs which are not expected to change are considered irrelevant costs and are excluded from analysis

Methods (Cont’d) 4. Relevant costs considered w Cost of vaccine (increase) w Cost of vaccine storage and transportation (decrease) w Cost of vaccination supplies (increase) w Transportation cost of supplies (increase) w Cost of sterilization and wastage disposal (increase) w Cost of training (start-up training for IPV delivery) w Labor cost (increase: longer time/injection, increased use of trained health workers) w Cost of vaccination visit (decrease: because of reduction in the No. of visits)

Methods (Cont’d) 5. Major data and assumptions w w w The No. of target children for OPV and IPV NID The No. of doses of OPV & IPV per FIC Coverage of OPV and IPV for different countries Vaccine wastage rate Price of vaccine (OPV & IPV) Labor costs for delivering OPV & IPV Costs of vaccination supplies Costs of vaccine storage and transport Number of vaccination visits needed Start-up training cost for IPV introduction

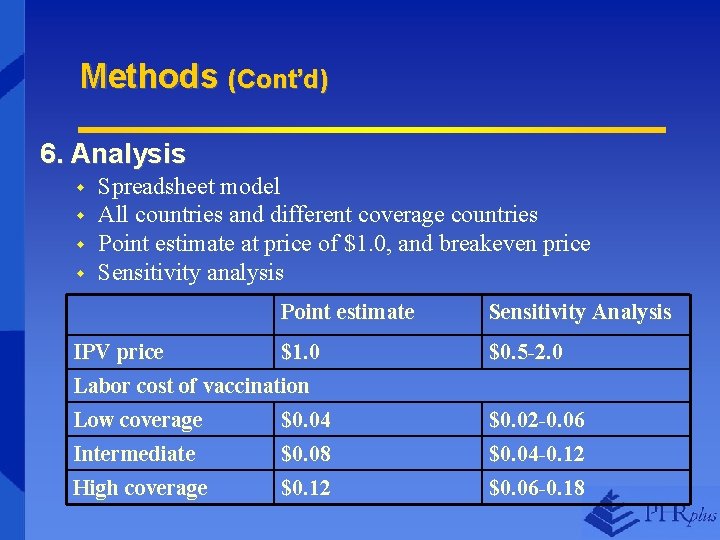
Methods (Cont’d) 6. Analysis w w Spreadsheet model All countries and different coverage countries Point estimate at price of $1. 0, and breakeven price Sensitivity analysis Point estimate Sensitivity Analysis IPV price $1. 0 Labor cost of vaccination $0. 5 -2. 0 Low coverage Intermediate $0. 04 $0. 08 $0. 02 -0. 06 $0. 04 -0. 12 High coverage $0. 12 $0. 06 -0. 18

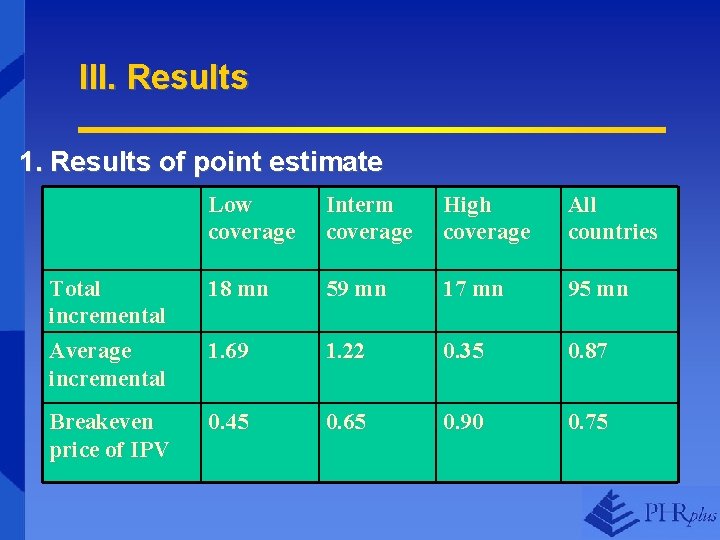
III. Results 1. Results of point estimate Low coverage Interm coverage High coverage All countries Total incremental Average incremental 18 mn 59 mn 17 mn 95 mn 1. 69 1. 22 0. 35 0. 87 Breakeven price of IPV 0. 45 0. 65 0. 90 0. 75

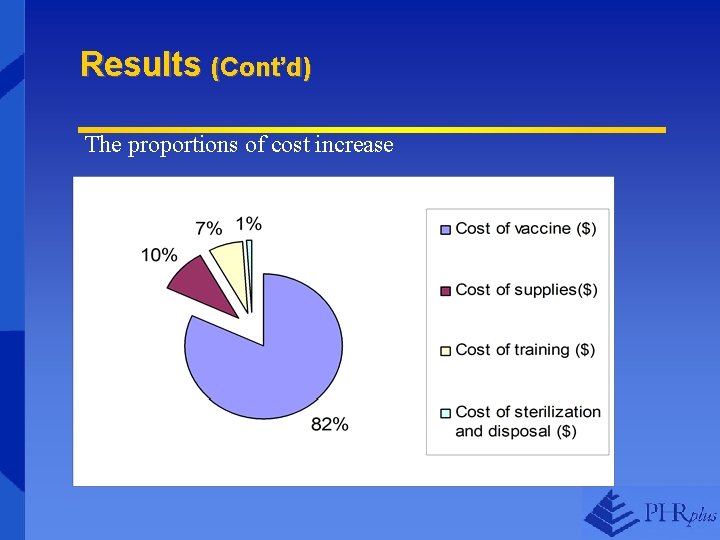
Results (Cont’d) The proportions of cost increase

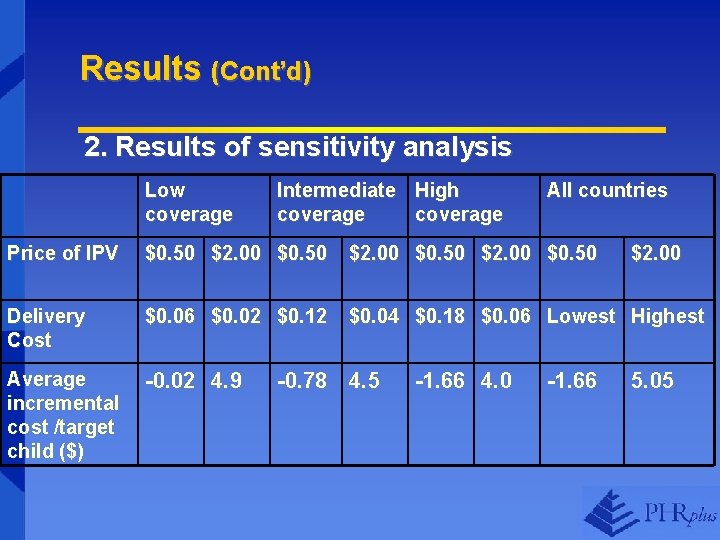
Results (Cont’d) 2. Results of sensitivity analysis Low coverage Intermediate High coverage All countries Price of IPV $0. 50 $2. 00 $0. 50 Delivery Cost $0. 06 $0. 02 $0. 12 $0. 04 $0. 18 $0. 06 Lowest Highest Average incremental cost /target child ($) -0. 02 4. 9 -0. 78 4. 5 -1. 66 4. 0 -1. 66 $2. 00 5. 05

Discussion w w Careful interpretation of results because of key assumptions -- IPV price and labor cost/visit Additional data are needed for decision-making w w Potential supply of IPV (production capacity & willingness to produce) Costs averted due to elimination of VAPP Increased risk of outbreak due to vaccine switch (lower coverage, vaccine virus revert, reduction in community protection, wild virus escape) Possible use of vaccine combos (price may be higher, but no extra injections, possible savings of logistic costs)

Conclusions w w The major factor that drives cost down is the reduction of the number of vaccination visits On average, the IPV price has to be reduced to less than $0. 75 to make a vaccine switch breakeven High coverage countries tend to have lower incremental cost and higher affordability, suggesting that they are priority countries for the switch Besides cost, other factors need to be considered in the decision to choose vaccines (as discussed)

Thank You The PHRplus Project is funded by U. S. Agency for International Development and implemented by: Abt Associates Inc. and partners, Development Associates, Inc. ; Emory University Rollins School of Public Health; Philoxenia International Travel, Inc. Program for Appropriate Technology in Health; SAG Corp. ; Social Sectors Development Strategies, Inc. ; Training Resources Group; Tulane University School of Public Health and Tropical Medicine; University Research Co. , LLC.
 Weed definition in agriculture
Weed definition in agriculture National leprosy eradication programme ppt
National leprosy eradication programme ppt Containment eradication
Containment eradication H pylori eradication therapy
H pylori eradication therapy After me after me after me
After me after me after me John 14 1-3
John 14 1-3 Lecture tubertest
Lecture tubertest Live vaccines mnemonic
Live vaccines mnemonic Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Edible vaccines pros and cons
Edible vaccines pros and cons Could vaccines breed viciousness
Could vaccines breed viciousness Www.cdc.gov/vaccines/schedules/index.html
Www.cdc.gov/vaccines/schedules/index.html Glyphosate in vaccines
Glyphosate in vaccines Brighton collaboration case definitions for vaccines
Brighton collaboration case definitions for vaccines