CHEMISTRY REVISION TOPIC 3 Quantative Chemistry What is
- Slides: 2

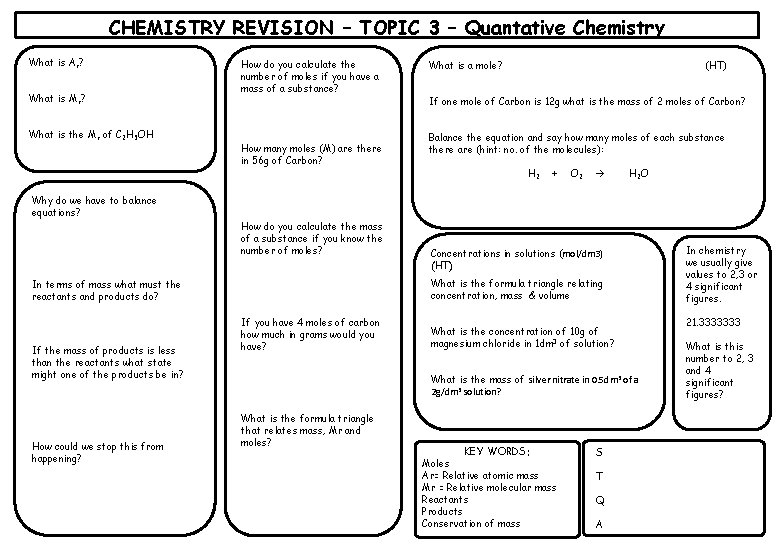
CHEMISTRY REVISION – TOPIC 3 – Quantative Chemistry What is Ar? What is Mr? What is the Mr of C 2 H 5 OH Why do we have to balance equations? How do you calculate the number of moles if you have a mass of a substance? How many moles (M) are there in 56 g of Carbon? How do you calculate the mass of a substance if you know the number of moles? How could we stop this from happening? (HT) If one mole of Carbon is 12 g what is the mass of 2 moles of Carbon? Balance the equation and say how many moles of each substance there are (hint: no. of the molecules): H 2 + O 2 H 2 O Concentrations in solutions (mol/dm 3) (HT) What is the formula triangle relating concentration, mass & volume In terms of mass what must the reactants and products do? If the mass of products is less than the reactants what state might one of the products be in? What is a mole? If you have 4 moles of carbon how much in grams would you have? What is the concentration of 10 g of magnesium chloride in 1 dm 3 of solution? What is the mass of silver nitrate in 0. 5 dm 3 of a 2 g/dm 3 solution? What is the formula triangle that relates mass, Mr and moles? KEY WORDS: Moles Ar= Relative atomic mass Mr = Relative molecular mass Reactants Products Conservation of mass S T Q A In chemistry we usually give values to 2, 3 or 4 significant figures. 21. 3333333 What is this number to 2, 3 and 4 significant figures?

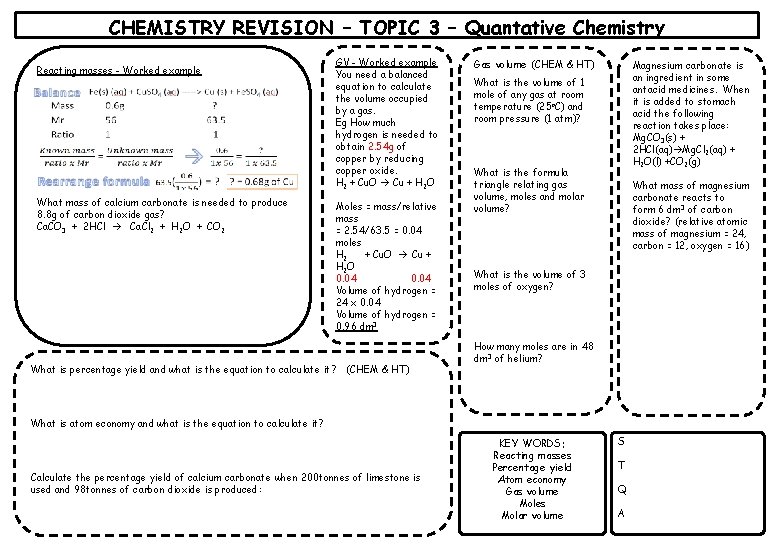
CHEMISTRY REVISION – TOPIC 3 – Quantative Chemistry Reacting masses - Worked example What mass of calcium carbonate is needed to produce 8. 8 g of carbon dioxide gas? Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 O + CO 2 GV - Worked example You need a balanced equation to calculate the volume occupied by a gas. Eg How much hydrogen is needed to obtain 2. 54 g of copper by reducing copper oxide. H 2 + Cu. O Cu + H 2 O Moles = mass/relative mass = 2. 54/63. 5 = 0. 04 moles H 2 + Cu. O Cu + H 2 O 0. 04 Volume of hydrogen = 24 x 0. 04 Volume of hydrogen = 0. 96 dm 3 What is percentage yield and what is the equation to calculate it? (CHEM & HT) Gas volume (CHEM & HT) Magnesium carbonate is an ingredient in some antacid medicines. When it is added to stomach acid the following reaction takes place: Mg. CO 3(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2 O(l) +CO 2(g) What is the volume of 1 mole of any gas at room temperature (25 o. C) and room pressure (1 atm)? What is the formula triangle relating gas volume, moles and molar volume? What mass of magnesium carbonate reacts to form 6 dm 3 of carbon dioxide? (relative atomic mass of magnesium = 24, carbon = 12, oxygen = 16) What is the volume of 3 moles of oxygen? How many moles are in 48 dm 3 of helium? What is atom economy and what is the equation to calculate it? Calculate the percentage yield of calcium carbonate when 200 tonnes of limestone is used and 98 tonnes of carbon dioxide is produced: KEY WORDS: Reacting masses Percentage yield Atom economy Gas volume Moles Molar volume S T Q A