Chemistry 1011 TOPIC Rate of Reaction TEXT REFERENCE


- Slides: 8

Chemistry 1011 TOPIC Rate of Reaction TEXT REFERENCE Masterton and Hurley Chapter 11 Chemistry 1011 Slot 5 1

11. 5 Catalysis YOU ARE EXPECTED TO BE ABLE TO: • Identify heterogeneous and homogeneous catalysts in chemical and biological systems. • Describe heterogeneous and homogeneous catalytic processes. • Interpret catalysis in terms of the effect on activation energy. Chemistry 1011 Slot 5 2

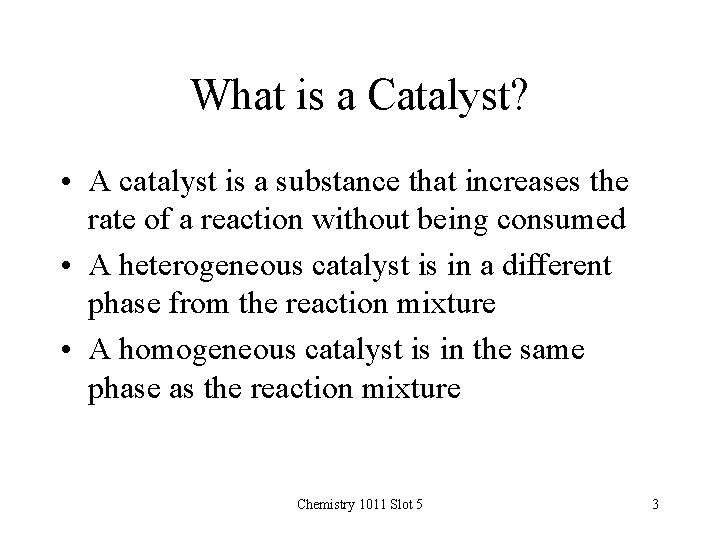
What is a Catalyst? • A catalyst is a substance that increases the rate of a reaction without being consumed • A heterogeneous catalyst is in a different phase from the reaction mixture • A homogeneous catalyst is in the same phase as the reaction mixture Chemistry 1011 Slot 5 3

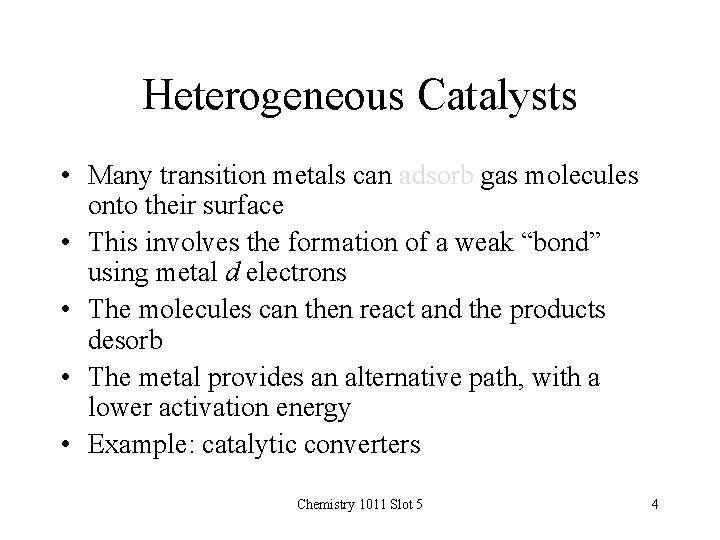
Heterogeneous Catalysts • Many transition metals can adsorb gas molecules onto their surface • This involves the formation of a weak “bond” using metal d electrons • The molecules can then react and the products desorb • The metal provides an alternative path, with a lower activation energy • Example: catalytic converters Chemistry 1011 Slot 5 4

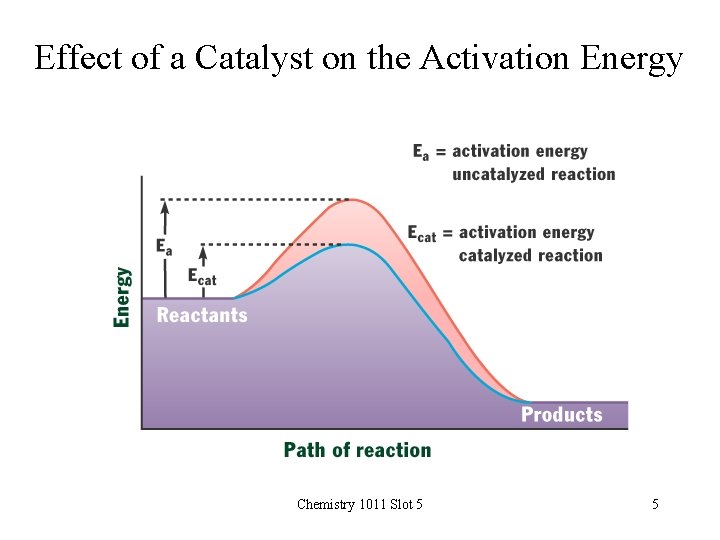
Effect of a Catalyst on the Activation Energy Chemistry 1011 Slot 5 5

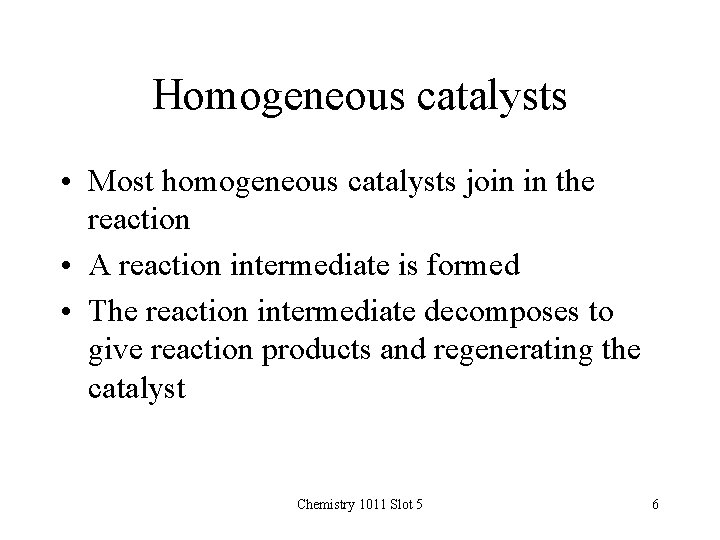
Homogeneous catalysts • Most homogeneous catalysts join in the reaction • A reaction intermediate is formed • The reaction intermediate decomposes to give reaction products and regenerating the catalyst Chemistry 1011 Slot 5 6

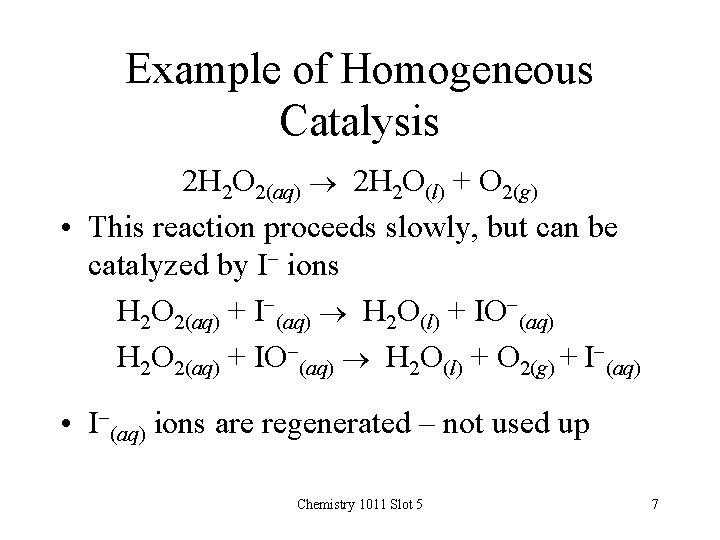
Example of Homogeneous Catalysis 2 H 2 O 2(aq) 2 H 2 O(l) + O 2(g) • This reaction proceeds slowly, but can be catalyzed by I- ions H 2 O 2(aq) + I-(aq) H 2 O(l) + IO-(aq) H 2 O 2(aq) + IO-(aq) H 2 O(l) + O 2(g) + I-(aq) • I-(aq) ions are regenerated – not used up Chemistry 1011 Slot 5 7

Enzymes • Catalysts in living organisms are called enzymes • Many are proteins with high molar masses • Many enzymes are very specific – Eg: maltase will catalyze the hydrolysis of maltose to glucose • Most enzymes operate only over a very narrow temperature range Chemistry 1011 Slot 5 8