Arcis solutions for saliva sample prep Simplified prep
- Slides: 7

Arcis solutions for saliva sample prep Simplified prep for lateral flow and PCR analysis Arcis Biotechnology Limited T: +44 1925 607101 E: info@arcisbio. com W: www. arcisbio. com

Move to less invasive rapid-testing Limitations of swab and blood-based testing: • • Sampling Requires healthcare professionals Patient discomfort with invasive sampling Cost and availability of lancets and swabs Limits expansion of test programs Saliva enables: • • Avoidance of assisted sample collection No patient discomfort Elimination of lancets and swabs Rapid expansion of test programs Arcis enables: • • Conversion of lateral flow from swab or blood to saliva No lances, swabs, or technician-assisted sample collection 25/11/2020 Arcis Bio. Technology - Commercial in confidence 2

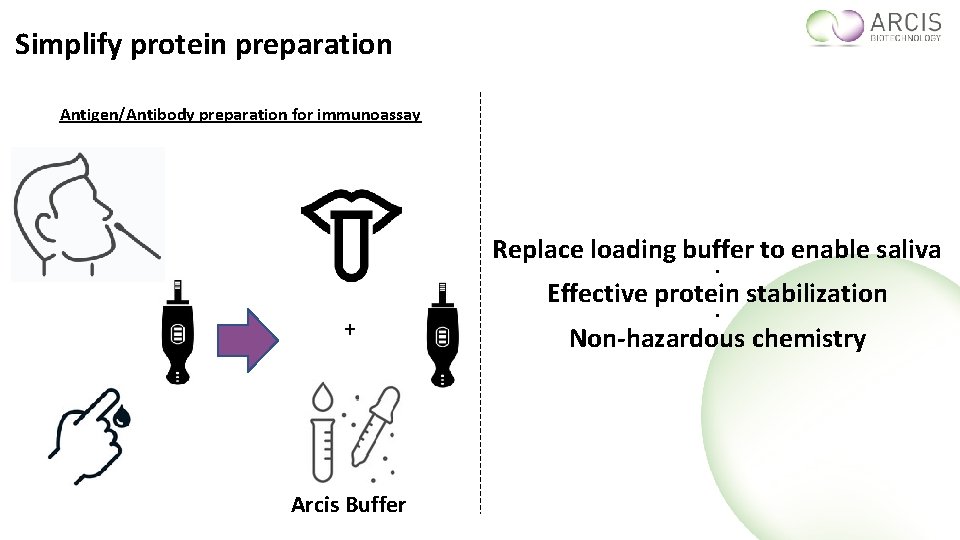
Simplify protein preparation Antigen/Antibody preparation for immunoassay Replace loading buffer to enable saliva ● Effective protein stabilization + Arcis Buffer ● Non-hazardous chemistry

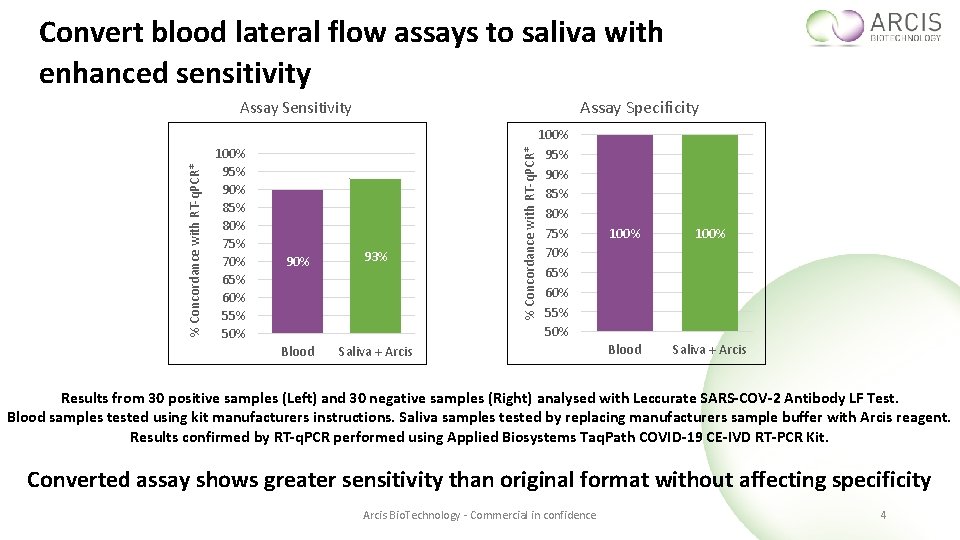
Convert blood lateral flow assays to saliva with enhanced sensitivity Assay Specificity 100% 95% 90% 85% 80% 75% 70% 65% 60% 55% 50% 93% Blood Saliva + Arcis % Concordance with RT-q. PCR* Assay Sensitivity 100% 95% 90% 85% 80% 75% 70% 65% 60% 55% 50% 100% Blood Saliva + Arcis Results from 30 positive samples (Left) and 30 negative samples (Right) analysed with Leccurate SARS-COV-2 Antibody LF Test. Blood samples tested using kit manufacturers instructions. Saliva samples tested by replacing manufacturers sample buffer with Arcis reagent. Results confirmed by RT-q. PCR performed using Applied Biosystems Taq. Path COVID-19 CE-IVD RT-PCR Kit. Converted assay shows greater sensitivity than original format without affecting specificity Arcis Bio. Technology - Commercial in confidence 4

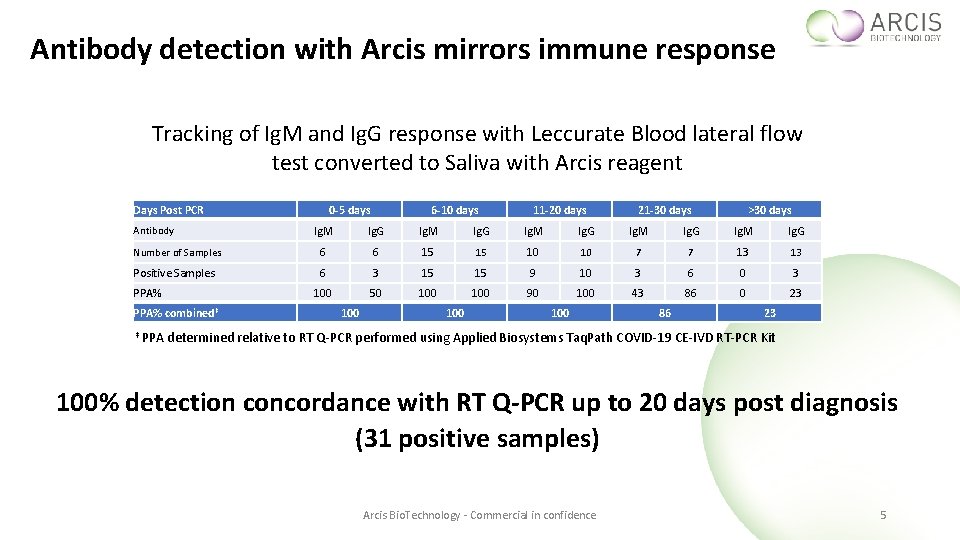
Antibody detection with Arcis mirrors immune response Tracking of Ig. M and Ig. G response with Leccurate Blood lateral flow test converted to Saliva with Arcis reagent Days Post PCR 0 -5 days 6 -10 days 11 -20 days 21 -30 days >30 days Ig. M Ig. G Number of Samples 6 6 15 15 10 10 7 7 13 13 Positive Samples 6 3 15 15 9 10 3 6 0 3 100 50 100 90 100 43 86 0 23 Antibody PPA% combined‡ ‡ PPA 100 100 86 23 determined relative to RT Q-PCR performed using Applied Biosystems Taq. Path COVID-19 CE-IVD RT-PCR Kit 100% detection concordance with RT Q-PCR up to 20 days post diagnosis (31 positive samples) Arcis Bio. Technology - Commercial in confidence 5

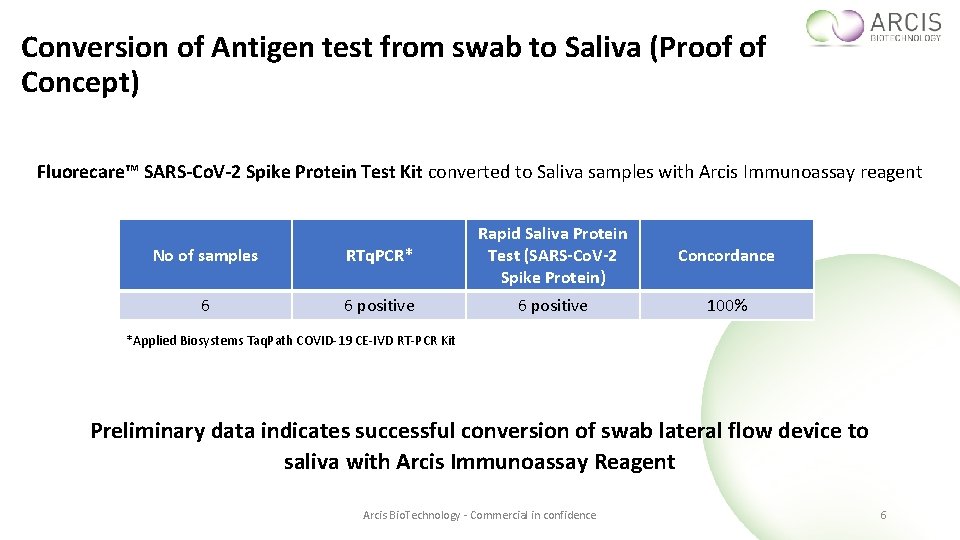
Conversion of Antigen test from swab to Saliva (Proof of Concept) Fluorecare™ SARS-Co. V-2 Spike Protein Test Kit converted to Saliva samples with Arcis Immunoassay reagent No of samples RTq. PCR* Rapid Saliva Protein Test (SARS-Co. V-2 Spike Protein) 6 6 positive Concordance 100% *Applied Biosystems Taq. Path COVID-19 CE-IVD RT-PCR Kit Preliminary data indicates successful conversion of swab lateral flow device to saliva with Arcis Immunoassay Reagent Arcis Bio. Technology - Commercial in confidence 6

Summary of ability to prep saliva for antigen and antibody testing Arcis results indicate ability to: • • • Convert lateral flow rapid tests from swab or blood to saliva Lyse virus without denaturing proteins No sacrifice of sensitivity Seeking partner for advancing the application • Arcis Biotechnology founded 2010 • Patented chemistry • Scalable manufacturing facility 7