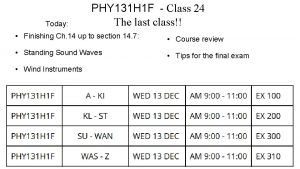
AljalalPhys 102 131 Ch 19 page 1 Questions

- Slides: 13

Aljalal-Phys 102 -131 -Ch 19 -page 1 Questions Chapter 19 The Kinetic Theory of Gases

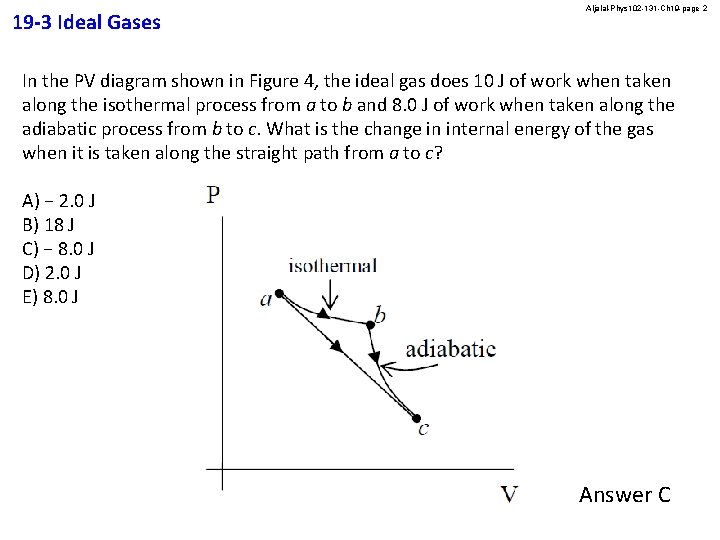
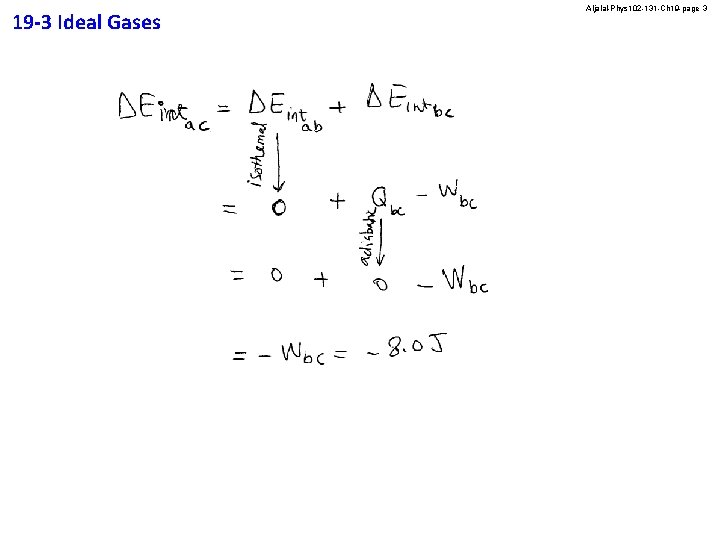
19 -3 Ideal Gases Aljalal-Phys 102 -131 -Ch 19 -page 2 In the PV diagram shown in Figure 4, the ideal gas does 10 J of work when taken along the isothermal process from a to b and 8. 0 J of work when taken along the adiabatic process from b to c. What is the change in internal energy of the gas when it is taken along the straight path from a to c? A) − 2. 0 J B) 18 J C) − 8. 0 J D) 2. 0 J E) 8. 0 J Answer C

19 -3 Ideal Gases Aljalal-Phys 102 -131 -Ch 19 -page 3

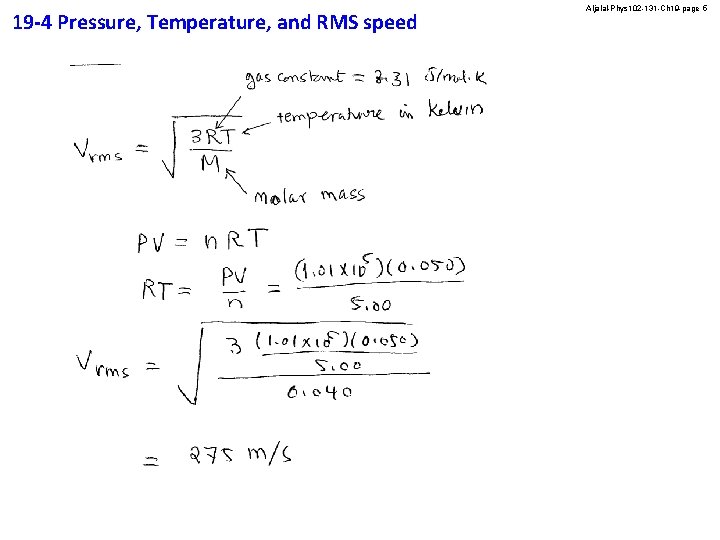
19 -4 Pressure, Temperature, and RMS speed Aljalal-Phys 102 -131 -Ch 19 -page 4 A 0. 050 -m 3 container has 5. 00 moles of argon gas at a pressure of 1. 00 atm. What is the rms speed of the argon molecules? (MAr = 40. 0 g/mole) A) 496 m/s B) 275 m/s C) 398 m/s D) 940 m/s E) 870 m/s Answer B

19 -4 Pressure, Temperature, and RMS speed Aljalal-Phys 102 -131 -Ch 19 -page 5

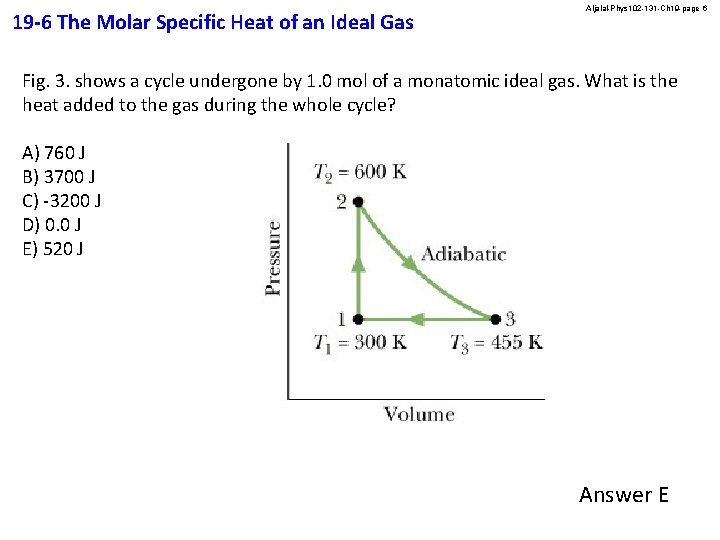
19 -6 The Molar Specific Heat of an Ideal Gas Aljalal-Phys 102 -131 -Ch 19 -page 6 Fig. 3. shows a cycle undergone by 1. 0 mol of a monatomic ideal gas. What is the heat added to the gas during the whole cycle? A) 760 J B) 3700 J C) -3200 J D) 0. 0 J E) 520 J Answer E

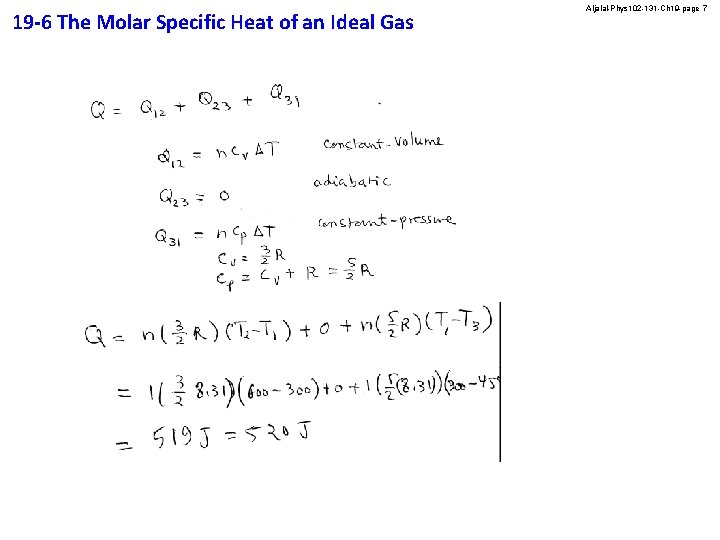
19 -6 The Molar Specific Heat of an Ideal Gas Aljalal-Phys 102 -131 -Ch 19 -page 7

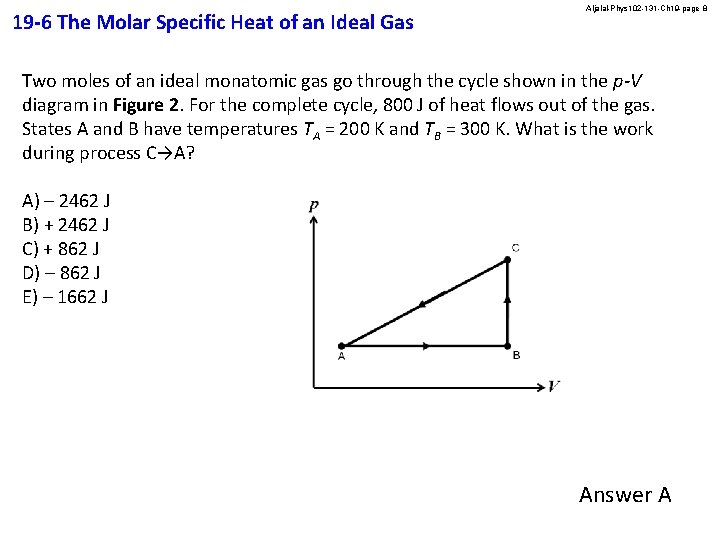
19 -6 The Molar Specific Heat of an Ideal Gas Aljalal-Phys 102 -131 -Ch 19 -page 8 Two moles of an ideal monatomic gas go through the cycle shown in the p-V diagram in Figure 2. For the complete cycle, 800 J of heat flows out of the gas. States A and B have temperatures TA = 200 K and TB = 300 K. What is the work during process C→A? A) – 2462 J B) + 2462 J C) + 862 J D) – 862 J E) – 1662 J Answer A

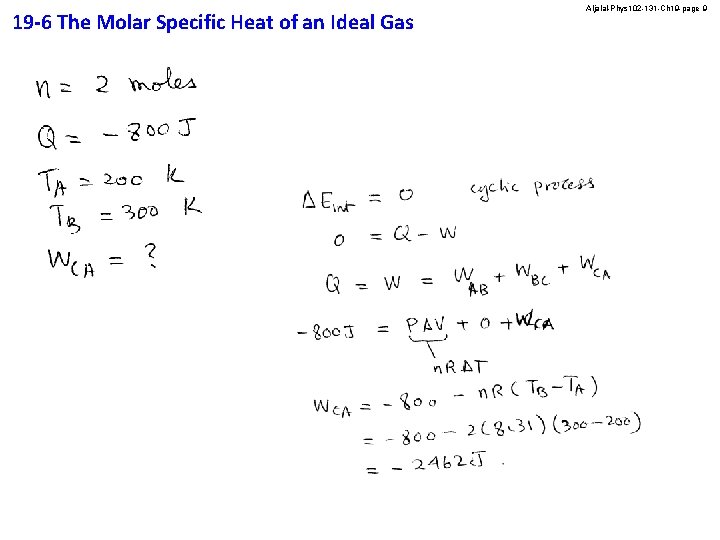
19 -6 The Molar Specific Heat of an Ideal Gas Aljalal-Phys 102 -131 -Ch 19 -page 9

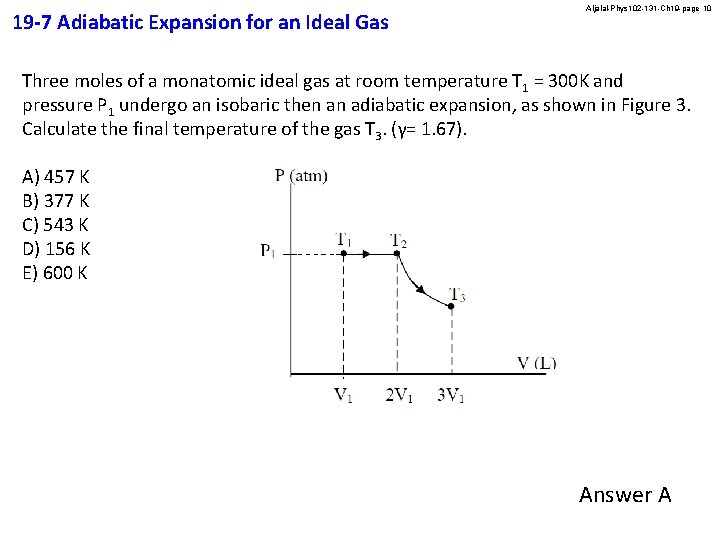
19 -7 Adiabatic Expansion for an Ideal Gas Aljalal-Phys 102 -131 -Ch 19 -page 10 Three moles of a monatomic ideal gas at room temperature T 1 = 300 K and pressure P 1 undergo an isobaric then an adiabatic expansion, as shown in Figure 3. Calculate the final temperature of the gas T 3. (γ= 1. 67). A) 457 K B) 377 K C) 543 K D) 156 K E) 600 K Answer A

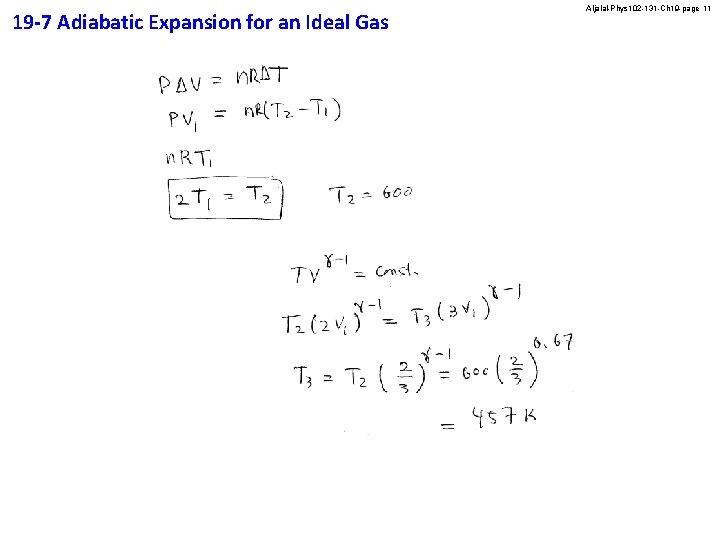
19 -7 Adiabatic Expansion for an Ideal Gas Aljalal-Phys 102 -131 -Ch 19 -page 11

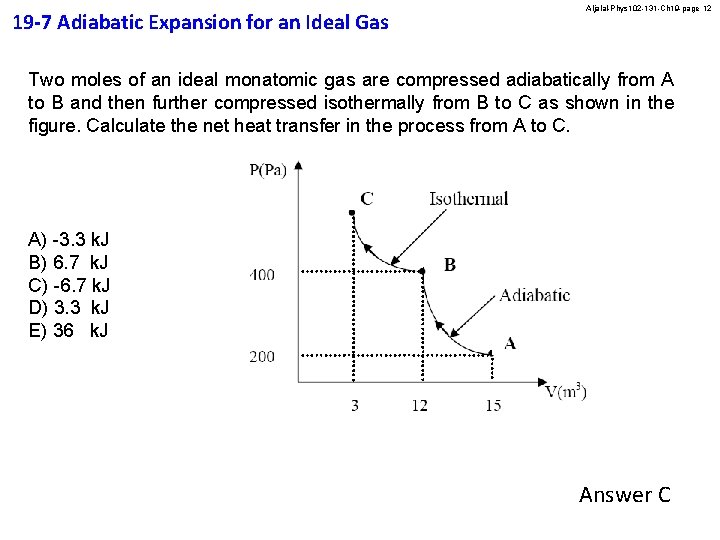
19 -7 Adiabatic Expansion for an Ideal Gas Aljalal-Phys 102 -131 -Ch 19 -page 12 Two moles of an ideal monatomic gas are compressed adiabatically from A to B and then further compressed isothermally from B to C as shown in the figure. Calculate the net heat transfer in the process from A to C. A) -3. 3 k. J B) 6. 7 k. J C) -6. 7 k. J D) 3. 3 k. J E) 36 k. J Answer C

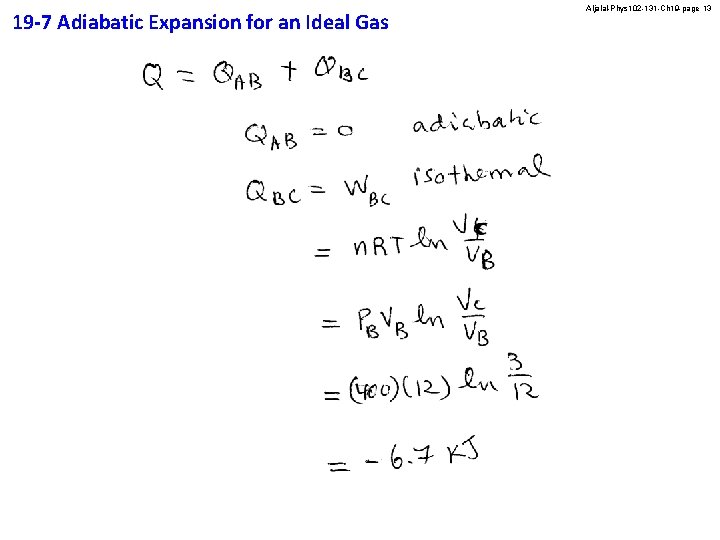
19 -7 Adiabatic Expansion for an Ideal Gas Aljalal-Phys 102 -131 -Ch 19 -page 13