Alirshad secondary school Form three classes Alirshad secondary










- Slides: 17

Al-irshad secondary school Form three classes

Al-irshad secondary school • Program: al-irshad online education • school: secondary school • Class: form three • Subject: chemistry • module 4: Non-metals • Lesson 5 : Ammonia • Teacher’s name: Abdirizak A/Lahi Alim

module 4: Non-metals Contents • • • Non-metals: Hydrogen, nitrogen Hydrogen nitrogen Making ammonia in industry Fertilisers Non-metals: Oxygen, Sulfur and Halogens Oxygen and oxides Sulfur and sulfur dioxide Sulfuric acid Chlorine and its compounds Bromine and iodine

Ammonia Lesson Objectives: By the end of this lesson, you should be able to: • Describe the properties and uses of ammonia • Prepare ammonia gas in the laboratory • Clarify the basicity of ammonia

Ammonia • Ammonia is a gas with the formula NH 3. • It is a very important compound, because it is used to make fertilisers. It is made in industry by reacting nitrogen with hydrogen. The details are in the next unit.

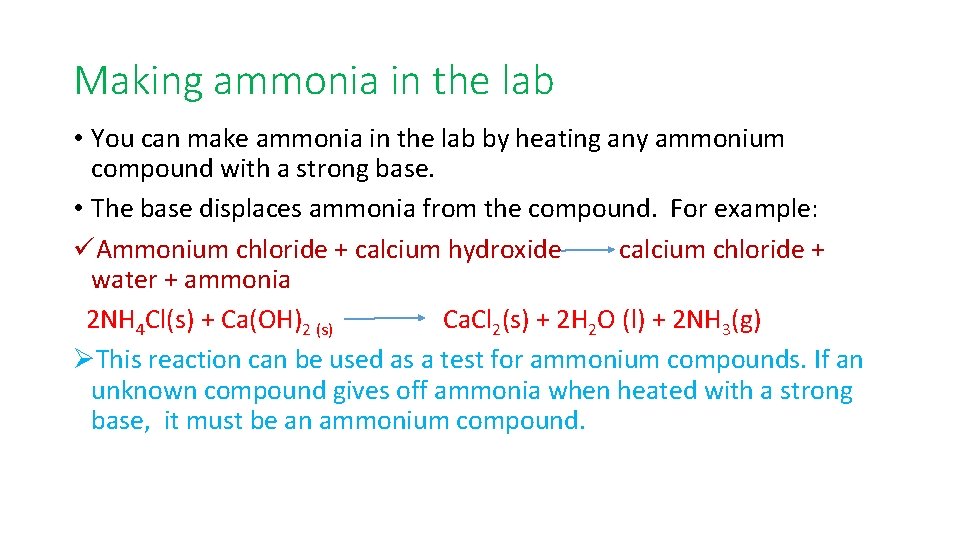
Making ammonia in the lab • You can make ammonia in the lab by heating any ammonium compound with a strong base. • The base displaces ammonia from the compound. For example: üAmmonium chloride + calcium hydroxide calcium chloride + water + ammonia 2 NH 4 Cl(s) + Ca(OH)2 (s) Ca. Cl 2(s) + 2 H 2 O (l) + 2 NH 3(g) ØThis reaction can be used as a test for ammonium compounds. If an unknown compound gives off ammonia when heated with a strong base, it must be an ammonium compound.

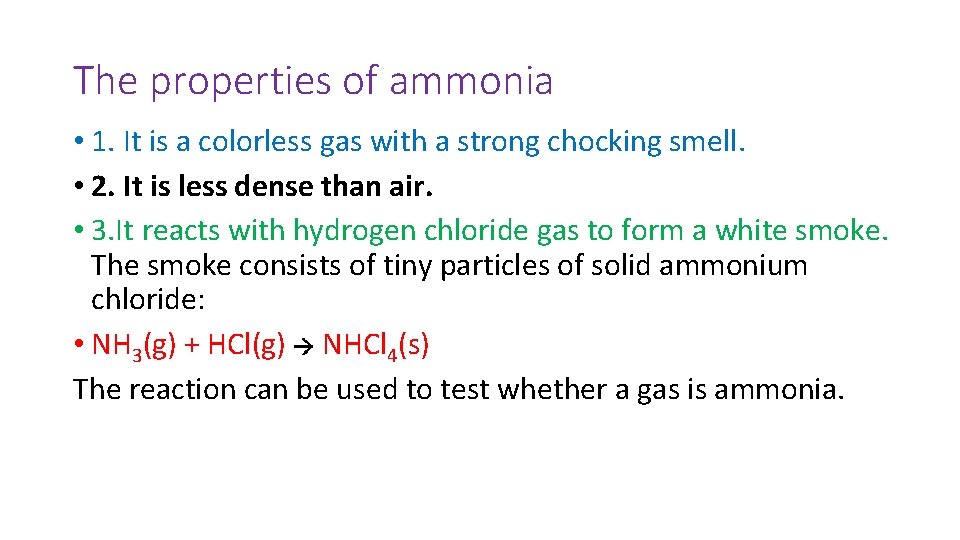
The properties of ammonia • 1. It is a colorless gas with a strong chocking smell. • 2. It is less dense than air. • 3. It reacts with hydrogen chloride gas to form a white smoke. The smoke consists of tiny particles of solid ammonium chloride: • NH 3(g) + HCl(g) NHCl 4(s) The reaction can be used to test whether a gas is ammonia.

5. It is very soluble in water. 6. The solution turns red litmus blue—it is alkaline. That means it contains hydroxide ions. Some of the ammonia has reacted with water to form ammonium ions and hydroxide ions: • NH 3 (g) + H 2 O(l) NH 4+ (aq)+ OH-(aq)

7. Since ammonia solution is alkaline, it reacts with acids to form salts. For example, with nitric acid it forms ammonium nitrate. Ammonium nitrate is important fertilizer. • NH 3 (g) + HNO 3 (aq) NH 4 NO 3 (aq)

Making ammonia in industry • In industry, ammonia is made from nitrogen and hydrogen in a process called the Haber process.

Obtaining the reactants Obtaining Hydrogen v. The hydrogen needed in the Haber process is obtained from the reaction between methane (North Sea gas) and steam. üCH 4(g) + H 2 O(g) → 3 H 2(g) + CO(g) • The carbon monoxide produced is then reacted with steam to produce more hydrogen gas. § CO(g) + H 2 O(g) → H 2(g) + CO 2(g) vor by cracking hydrocarbons Obtaining nitrogen v. The nitrogen needed in the Haber process is obtained from the atmosphere by fractional distillation of liquid air.

• The reaction between hydrogen and nitrogen is reversible. At a certain point, ammonia breaks down at the same rate as it forms. So conditions must be chosen to give the highest possible yield for the lowest cost. These conditions are: ü High pressure. ü Ammonia removed, so that more will form. üModerate heat and a catalyst.

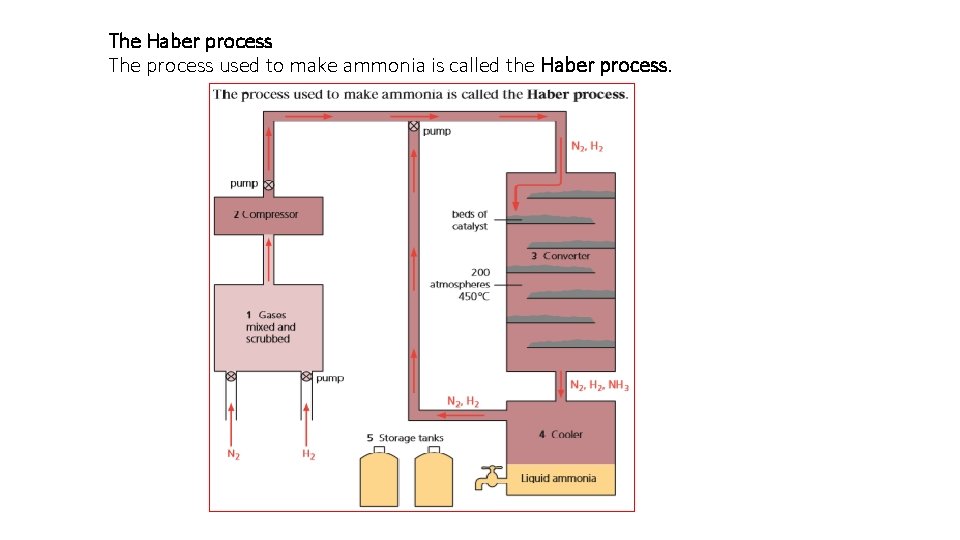
The Haber process The process used to make ammonia is called the Haber process.

These are the steps in Haber process: 1. The two gases are mixed, and the mixture is scrubbed to get rid of impurities. 2 The gas mixture is compressed. More and more gas is pumped in, until the pressure reaches 200 atmospheres. 3 The compressed gas flows to the converter – a round tank with beds of hot iron at 450°C. The iron catalyses the reversible reaction: üN 2 (g) + 3 H 2 (g) 2 NH 3 (g) • But only 15% of the mixture leaving the converter is ammonia.

4. The mixture is cooled until the ammonia condenses to a liquid. • The nitrogen and hydrogen are recycled to the converter for another chance to react. Steps 3 and 4 are continually repeated. 5. The ammonia is run into tanks, and stored as a liquid under pressure.

Uses of ammonia • It is used to make fertilizers such as ammonium nitrate and ammonium sulphate. • It is used to make household cleaners, dyes, explosives, and nylon. • A lot is used to make nitric acid.

 Classes e subclasses palavras
Classes e subclasses palavras Pre ap classes vs regular classes
Pre ap classes vs regular classes What are the three classes of yeast bread?
What are the three classes of yeast bread? Characteristics of flat worms
Characteristics of flat worms What is multigrade
What is multigrade Levers
Levers Flatworms
Flatworms Intruders in cryptography and network security
Intruders in cryptography and network security Consider the following three classes
Consider the following three classes Consider the following three classes
Consider the following three classes What are the three classes of mollusks
What are the three classes of mollusks Affirmative form present continuous
Affirmative form present continuous Ivc summer classes for high school students
Ivc summer classes for high school students How to make sabbath school interesting
How to make sabbath school interesting High school 101 class
High school 101 class Orc and hobbit problem
Orc and hobbit problem Othello act 3 summary
Othello act 3 summary In three minutes write three things
In three minutes write three things