Access to essential medicines for HIVAIDS update on







- Slides: 9

Access to essential medicines for HIV/AIDS update on WHO activities within UN access framework Peter Graaff Department of Essential Drugs and Medicines Policy Department of HIV World Health Organization

Selection 1. Rational selection and use 2002: n WHO guidelines for a public health approach to on scaling up antiretroviral therapy in resource limited settings : http: //www. who. int/HIV ä ä n standardized and simplified regimens simplified patient monitoring 12 ARVs on WHO Model EML & WMF 2003 (planned) n Update and prioritization ? Global stand. 1 st line Access HIV 2 Department of Essential Drugs and Medicines Policy

Affordable prices 2. Affordable prices for governments, healthcare providers and consumers - strategies: n National policy ä ä ä n Market dynamics ä n exemption from taxes, import duties price regulation (producer prices, distribution margins) WTO/TRIPs provisions generic competition Procurement management ä ä price information patent status and regulatory status price negotiation group purchasing (public, NGO - national, regional) Access HIV 3 Department of Essential Drugs and Medicines Policy

Affordable prices Indicative price information promotes transparency and competition n MSH-WHO essential drugs price indicator n UNICEF, UN-AIDS, WHO Sources and Prices (selected medicines and diagnostics) n Pharmaceutical starting materials n Antiretroviral drugs in the Americas n AFRO Essential Drugs Access HIV 4 Department of Essential Drugs and Medicines Policy

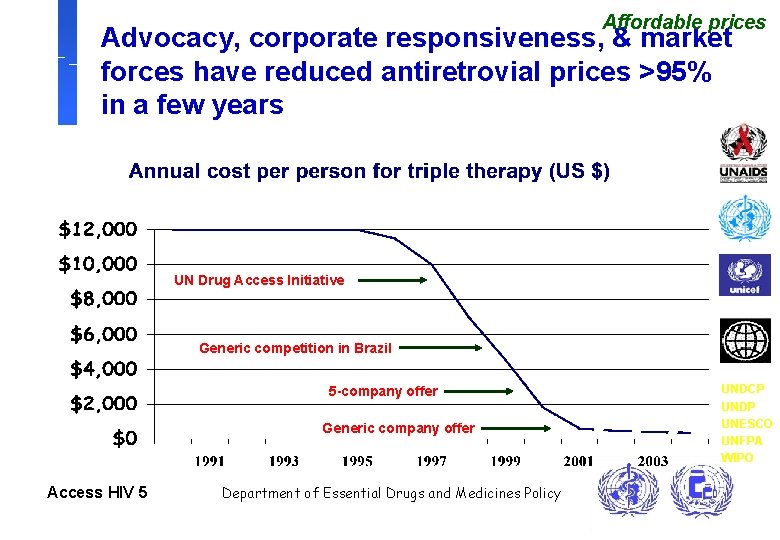
Affordable prices Advocacy, corporate responsiveness, & market forces have reduced antiretrovial prices >95% in a few years UN Drug Access Initiative Generic competition in Brazil 5 -company offer Generic company offer Access HIV 5 Department of Essential Drugs and Medicines Policy UNDCP UNDP UNESCO UNFPA WIPO

Financing 3. Sustainable financing In over 38 countries public drug expenditures are <US$2 per capita - inadequate by most estimates Key actions: n n n Increase public funding for cost-effective drugs Expand drug benefits in health insurance Seek external funding for the poorest populations (e. g. Global Fund) NB: This “leg” of the access table needs to be strengthened Access HIV 6 Department of Essential Drugs and Medicines Policy

Reliable systems Pilot Procurement, Quality and Sourcing Project n Objective: ä ä n Partners: ä n Establish a Model Quality Assurance System for Procurement of Pharmaceuticals Pre-qualification of suppliers of HIV/AIDS-related pharmaceutical products Quality standards of newer HIV medicines Improved capacity of national regulatory bodies UNAIDS, UNFPA, UNICEF, WHO, World Bank Products: ä 50 ARVs, 44 mono, 5 double and 1 triple combination Access HIV 7 Department of Essential Drugs and Medicines Policy

New developments ……. . 3 x 5 Scale up access to ARV to 3 million by 2005 n 300, 000 out of 6, 000 who should be on treatment in the developing world receive ARVs (Africa: 50, 000 out of 4, 000) n 22/09/03 emergency declaration n 01/12/03 World AIDS day. Present implementation plan to include: ä ä Intensified country support (priority countries) Tools (simplified) to move beyond capital and medical doctor “AIDS drug and diagnostic facility” ……. . Access HIV 8 Department of Essential Drugs and Medicines Policy

4. Summary n n UN, WHO and partners are active in all aspects of access to HIV/AIDS medicines Key tools available to governments and NGOs: Treatment guidelines and essential medicines list ä Price information ä Pre-qualified suppliers and products ä Patent status and regulatory status ä Guidance on use of TRIPS safeguards ä Policy and training tools in supply management (pricing, donations, procurement and supply management) ä n 3 x 5 should “affect” us all Access HIV 9 Department of Essential Drugs and Medicines Policy
 Deferred update and immediate update
Deferred update and immediate update Microsoft security essential tidak bisa update
Microsoft security essential tidak bisa update Characteristics of lipids
Characteristics of lipids Medicines information centre
Medicines information centre European directorate for the quality of medicines
European directorate for the quality of medicines Ggc medicines
Ggc medicines Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Medicines management programme
Medicines management programme Martindale medicines complete
Martindale medicines complete Which legislation helped solve trusts
Which legislation helped solve trusts

















