Access to Essential Medicines and Intellectual Property Rights




















- Slides: 25

Access to Essential Medicines and Intellectual Property Rights in Developing Countries Rachel M. Cohen Access to Essential Medicines Campaign Doctors Without Borders/Médecins Sans Frontières (MSF) 5 th Meeting of the Transatlantic Consumer Dialogue Workshop on Intellectual Property Rights: October 31, 2002

The Access to Medicines Crisis Communicable diseases = leading causes of death in developing countries, accounting for over 25% of all deaths globally 14 million die each year, 97% in developing countries - HIV/AIDS: 3 million per year - Tuberculosis: 2 million per year - Malaria: 1 -2 million per year 1/3 of world lacks access to essential quality drugs, diagnostics, vaccines

The Example of HIV/AIDS…

HIV/AIDS: The Price Barrier HIV/AIDS - 40 million people infected with HIV worldwide - Antiretroviral (ARV) therapy proven to dramatically extend and improve lives of people living with HIV/AIDS in wealthy countries, reducing AIDSrelated deaths by over 70%. - Average cost of antiretroviral “cocktail” in the US = $10, 000 -$15, 000 per patient per year - Over 95% of PWAs in developing countries lack access to ARVs, largely because of the price of drugs 8, 000 people with HIV/AIDS will die today

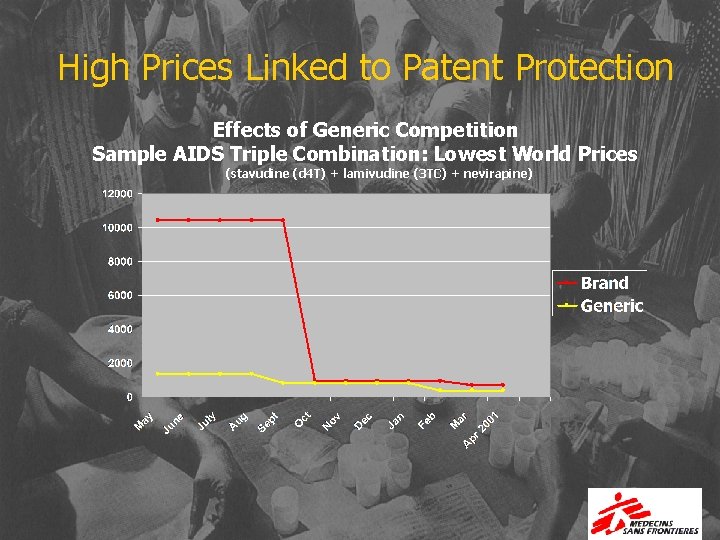
High Prices Linked to Patent Protection Effects of Generic Competition Sample AIDS Triple Combination: Lowest World Prices (stavudine (d 4 T) + lamivudine (3 TC) + nevirapine)

Best offers for first-line regimens proposed in the WHO guidelines October 2002

TRIPS Agreement: What Is It? World Trade Organization Agreement on Trade. Related Aspects of Intellectual Property Rights (1994 Uruguay Round) Most comprehensive international agreement on intellectual property rights such as patents, copyrights, and trademarks Sets forth minimum standards for intellectual property protection that must be met by all WTO Members (by 2005 at the latest)

TRIPS Agreement: What Is It? Research-based pharmaceutical companies key architects of TRIPS Medicines treated like any other commodity (Barbie dolls, computer software, CDs, etc. ) Commercial interests protected over public health

Patent Rights vs. Patient Rights

Correcting the Imbalance Impact of TRIPS is not fully visible today In years to come, new medicines invented since the signing of the TRIPS Agreement in 1994 will be potentially patentable in all WTO Member States depending on the will of the patent owner only Challenge to correct imbalance WTO Members can provide for a number of “public health” safeguards in national legislation to protect public health, e. g. in cases of patent abuses or emergencies

What Was Accomplished in Doha? The fundamental argument was settled: “We agree that the TRIPS Agreement does not and should not prevent Members from taking measures to protect public health. Accordingly, while reiterating our commitment to the TRIPS Agreement, we affirm that the Agreement can and should be interpreted and implemented in a manner supportive of WTO Members' right to protect public health and, in particular, to promote access to medicines for all. In this connection, we reaffirm the right of WTO Members to use, to the full, the provisions in the TRIPS Agreement, which provide flexibility for this purpose. ” - Ministerial Declaration on the TRIPS Agreement and Public Health November 2001

Unresolved Issues Paragraph 6: “production for export” => How countries with insufficient or no manufacturing capacity in the pharmaceutical sector will be able to make effective use of compulsory licensing under the TRIPS Agreement Paragraph 7: How to encourage and promote technology transfer to LDCs

The CIPR Report: IPRs & Health September 2002 Patents are tools of public policy and must operate to serve the greater public good. Developing countries should narrow to an absolute minimum the type and scope of pharmaceutical patents, and LDCs should consider delaying the granting of pharmaceutical patents for as long as possible. Need quick, easy-to-use measures to ensure generic competition to bring drug prices down in developing countries (e. g. compulsory licensing as rule rather than exception to ensure that patent system does not hamper the development of a competitive pharmaceutical market). Solution to production for export problem must be quick and easy to implement, give long term security and be economically viable (Article 30 approach). Patent system is failing to stimulate innovation to meet many medical needs, particularly the needs in developing countries. Further recognition of the need for greater action and support to help developing countries put health first.

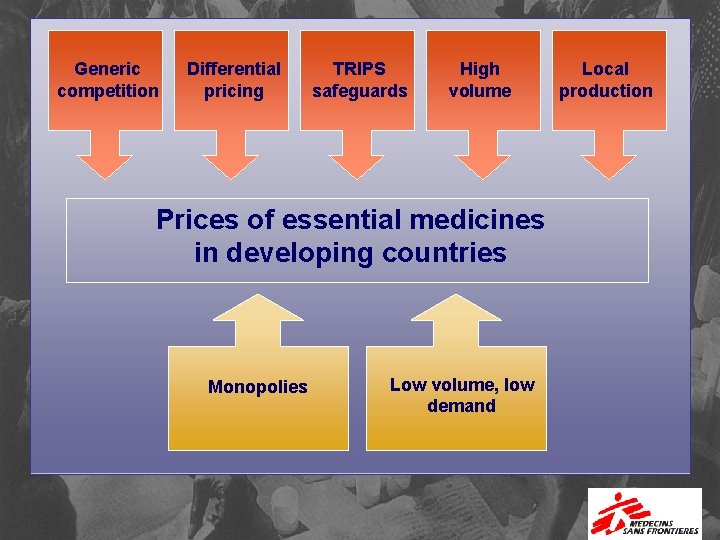
What More Is Needed for Existing Medicines? Equity pricing for equitable access…

Generic competition Differential pricing TRIPS safeguards High volume Prices of essential medicines in developing countries Monopolies Low volume, low demand Local production

What About Future Medicines? The Crisis in R&D for Neglected Diseases

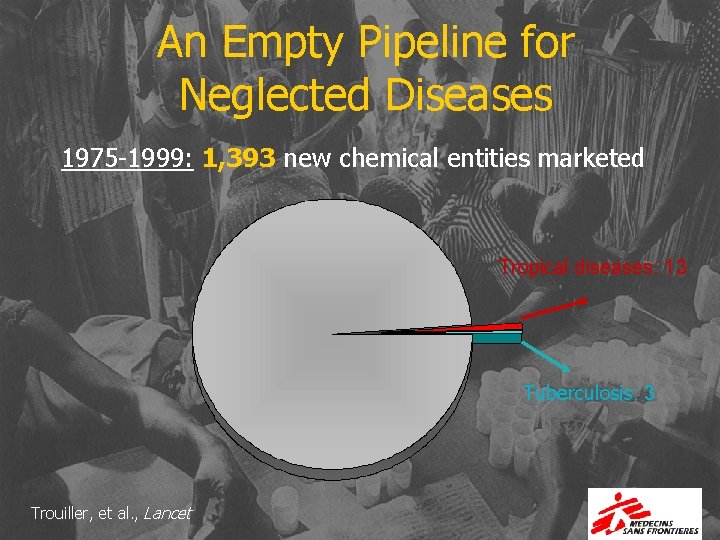
An Empty Pipeline for Neglected Diseases 1975 -1999: 1, 393 new chemical entities marketed Tropical diseases: 13 Tuberculosis: 3 Trouiller, et al. , Lancet

Will Strengthening IP Protection in Developing Countries Stimulate R&D for Diseases of the Poor?

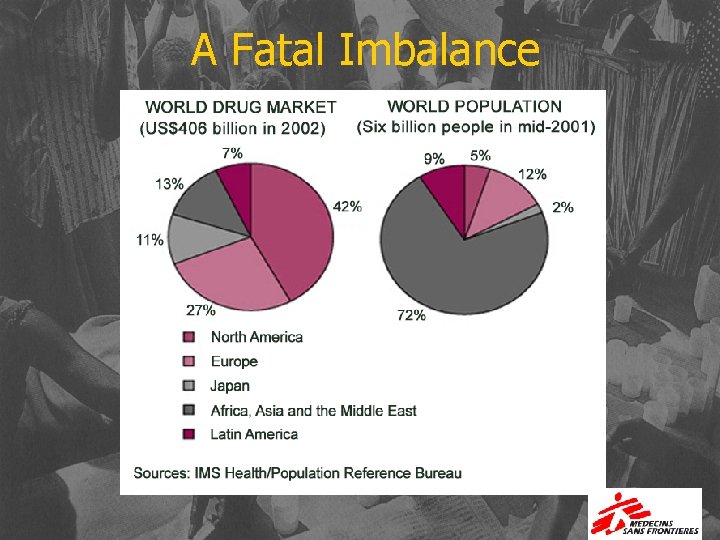
A Fatal Imbalance

Market Failure • Drug development is almost exclusively confined to the R&D-based pharmaceutical industry, operating in a global market economy • Market forces skew the direction of drug R&D towards those diseases and patients (consumers) that assure the highest financial returns • Market potential does not mirror health needs

Market Failure = Public Policy Failure Societal choice: drug development can be confined to the private sector Medicines are considered commodities that need to generate profit in return for private investment - public policy initiatives only stimulate R&D for diseases that affect wealthy markets - drug R&D capacity in developing countries is not adequately cultivated

What Kinds of Solutions Are Needed? • Market “push/pull mechanisms” mechanisms? Push: e. g. tax credits, R&D grants, support for clinical trials Pull: e. g. purchase funds, temporary monopolies, patent extensions • Public Private Partnerships (PPPs)? e. g. IAVI, GATB, MMV • WHO/UNDP/World Bank Special Program on Tropical Disease Research (TDR)? Very broad mandate, chronically under-funded, political constraints • A Drugs for Neglected Diseases Initiative (DNDi): Partnership for Public Response? Needs-based, field-driven, with clear public sector responsibility

What Kinds of Solutions Are Needed? Increased public sector funding for R&D for neglected diseases (including support for notfor-profit R&D) “Essential research obligation” (mandatory reinvestment of percentage of profits toward R&D for neglected diseases either directly or through public R&D programs) Global treaty on R&D for neglected diseases

Overcoming the Gap: Toward a New R&D Paradigm Ensuring access to medicines is a public sector responsibility (when the market fails, governments have a duty to step in) Must develop an R&D agenda that is needs-driven, not profit-driven Must acknowledge differences between neglected and most neglected diseases, and develop strategies accordingly

Thank You rachel. cohen@newyork. msf. org www. doctorswithoutborders. org www. accessmed-msf. org
 Trade-related aspects of intellectual property rights
Trade-related aspects of intellectual property rights Intellectual property rights in professional practices
Intellectual property rights in professional practices Intellectual property rights
Intellectual property rights Intellectual property rights
Intellectual property rights Importance of intellectual property
Importance of intellectual property Intellectual property management definition
Intellectual property management definition Licensing advantages
Licensing advantages Intellectual property business plan example
Intellectual property business plan example Property
Property Right to intellectual property of teachers
Right to intellectual property of teachers Intellectual property law definition
Intellectual property law definition Incentive theory
Incentive theory Concept of intellectual property
Concept of intellectual property Valuing intangible assets
Valuing intangible assets Characteristics of intellectual property
Characteristics of intellectual property Discuss intellectual property frankly
Discuss intellectual property frankly Intangible inputs
Intangible inputs Intellectual property
Intellectual property Intellectual property statement
Intellectual property statement Intellectual property business plan example
Intellectual property business plan example Evalueserve patent search
Evalueserve patent search Discuss intellectual property frankly
Discuss intellectual property frankly At&t ecommerce
At&t ecommerce Positive vs negative rights
Positive vs negative rights Duty towards self
Duty towards self Legal rights and moral rights
Legal rights and moral rights














































