1 3 Thinking Like a Scientist Chapter 1













- Slides: 16

1. 3 Thinking Like a Scientist > Chapter 1 Introduction to Chemistry 1. 1 The Scope of Chemistry 1. 2 Chemistry and You 1. 3 Thinking Like a Scientist 1. 4 Problem Solving in Chemistry 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > CHEMISTRY & YOU In 1928, Alexander Fleming, a Scottish scientist, noticed that the bacteria he was studying did not grow in the presence of a yellow-green mold. He thought the mold was killing the bacteria. How could Alexander Fleming tested his hypothesis? 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > An Experimental Approach to Science The word chemistry comes from the word alchemy. • Alchemists were concerned with searching for a way to change other metals, such as lead, into gold. • Alchemists developed the tools and techniques for working with chemicals. • They designed equipment that is still in use today, including beakers, flasks, tongs, funnels, and the mortar and pestle. 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > An Experimental Approach to Science How did Lavoisier help to transform chemistry? 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > An Experimental Approach to Science Lavoisier helped to transform chemistry from a science of observation (qualitative) to the science of measurement (quantitative) that it is today. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > An Experimental Approach to Science Antoine-Laurent Lavoisier did work in the late 1700 s that revolutionized the science of chemistry. • He designed a balance that could measure mass to the nearest 0. 0005 gram. • He settled a long-standing debate that oxygen is required for a material to burn. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

Who is credited with transforming chemistry from a science of observation to a science of measurement? A. Fleming B. Lavoisier C. de Mestral D. Carothers Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > The Scientific Method The scientific method is a logical, systematic approach to the solution of a scientific problem. * Chemists use the scientific method to solve problems and develop theories about the natural world. 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > The Scientific Method What are the steps in the scientific method? 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > The Scientific Method Steps in the scientific method include: 1. ) Question 2. ) Hypothesis 3. ) Test 4. ) Analyze Data 5. ) Conclusion 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > The Scientific Method Making Observations What is the difference between an Observation and an Inference? • An observation is only what your senses tell you. • An inference takes what you observe and adds judgment to it. 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > The Scientific Method Making Observations (Question) You try to start your car and you notice that it does not turn over while making a clicking sound. * You guess that the battery is dead. You are making a hypothesis. 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > The Scientific Method Testing Hypotheses • A hypothesis is a proposed TESTABLE explanation for an observation. • Comes in an “IF” and “THEN” statement. example: IF this is true, THEN this should happen. 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > The Scientific Method Testing Hypotheses Experiment, a organized procedure that is used to test a hypothesis. 1. ) charging the battery (just start with cables) 2. ) replacing the battery 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

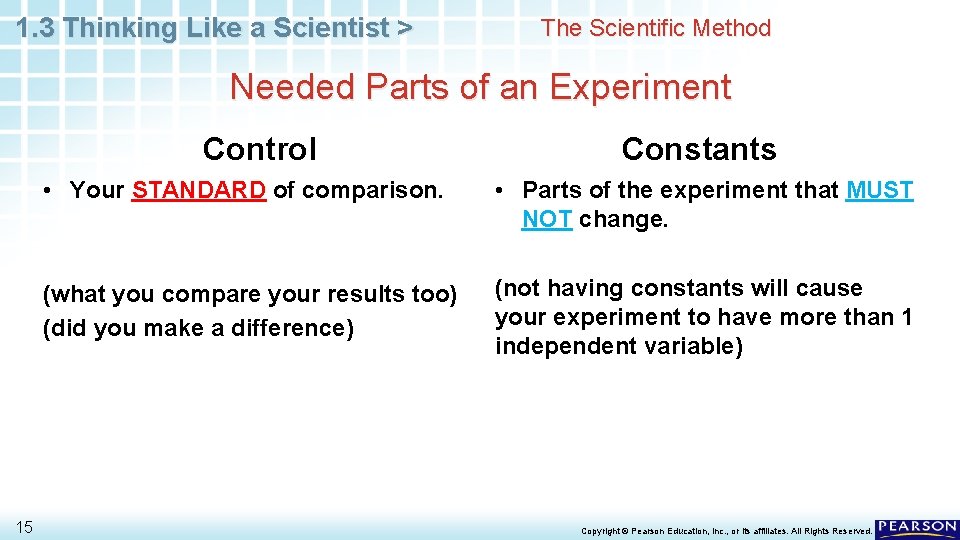
1. 3 Thinking Like a Scientist > The Scientific Method Needed Parts of an Experiment Control 15 Constants • Your STANDARD of comparison. • Parts of the experiment that MUST NOT change. (what you compare your results too) (did you make a difference) (not having constants will cause your experiment to have more than 1 independent variable) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

1. 3 Thinking Like a Scientist > END OF 1. 3 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.