Zymogenproenzyme inactive enzyme precursor Feed forward reaction positive


- Slides: 14

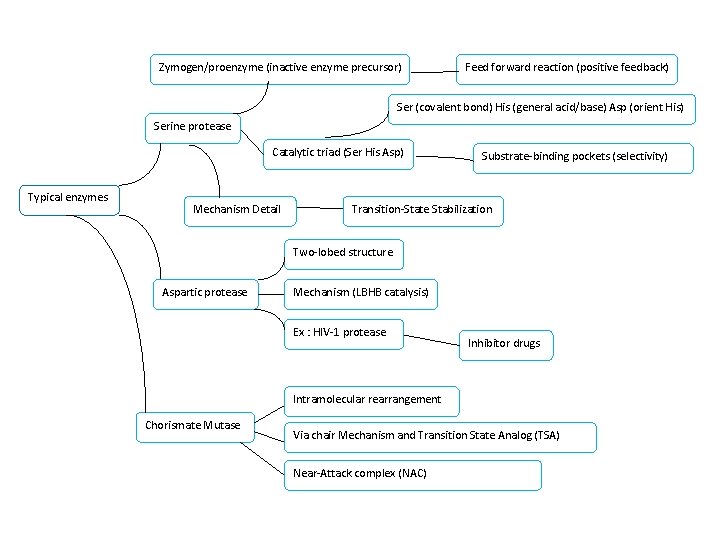
Zymogen/proenzyme (inactive enzyme precursor) Feed forward reaction (positive feedback) Ser (covalent bond) His (general acid/base) Asp (orient His) Serine protease Catalytic triad (Ser His Asp) Typical enzymes Mechanism Detail Substrate-binding pockets (selectivity) Transition-State Stabilization Two-lobed structure Aspartic protease Mechanism (LBHB catalysis) Ex : HIV-1 protease Inhibitor drugs Intramolecular rearrangement Chorismate Mutase Via chair Mechanism and Transition State Analog (TSA) Near-Attack complex (NAC)

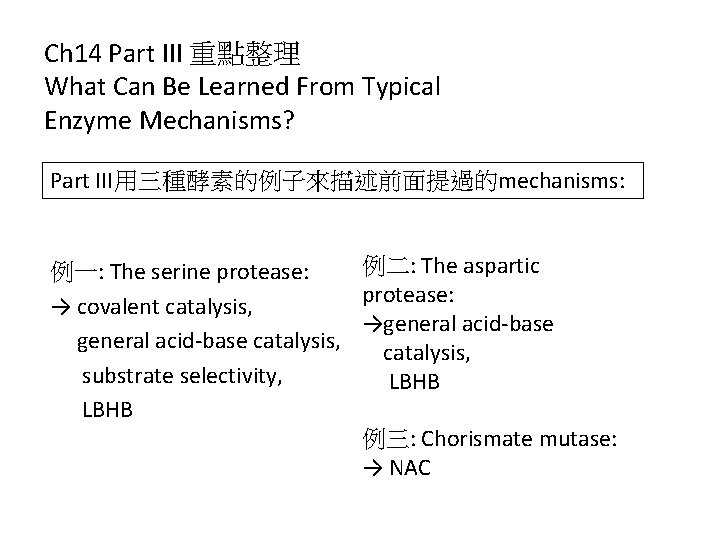
Ch 14 Part III 重點整理 What Can Be Learned From Typical Enzyme Mechanisms? Part III用三種酵素的例子來描述前面提過的mechanisms: 例二: The aspartic 例一: The serine protease: → covalent catalysis, →general acid-base catalysis, substrate selectivity, LBHB 例三: Chorismate mutase: → NAC

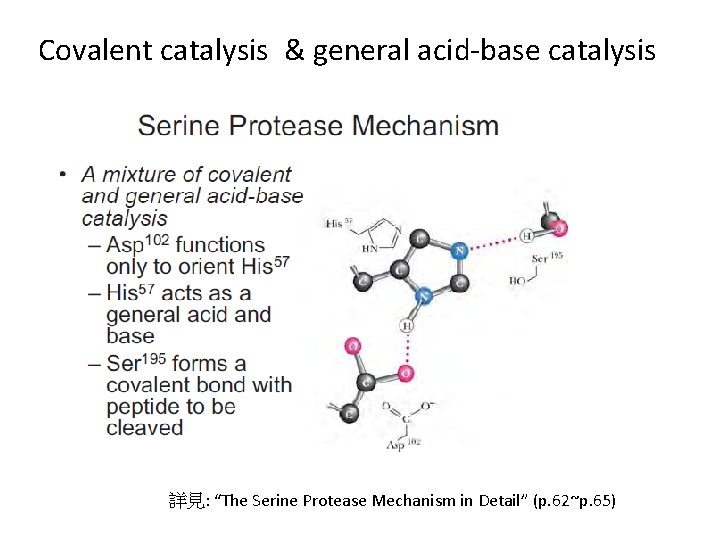
The serine protease: (以Ser為活性中心的蛋白酶) Serine protease family 包含: -消化道酵素: typsin, chymotrypsin, elastase (分泌時以zymogens型態出現) - 凝血蛋白酶: Thrombin (詳見Ch 15) Catalytic Triad (三個主要的活性中心) His 57, Asp 102, Ser 195 Zymogen (酵素前驅物) *Positive feedback 可自身活化 經水解反應 (hydrolysis) 或構形改變 (configuration change) 使active site 暴露 Active enzyme

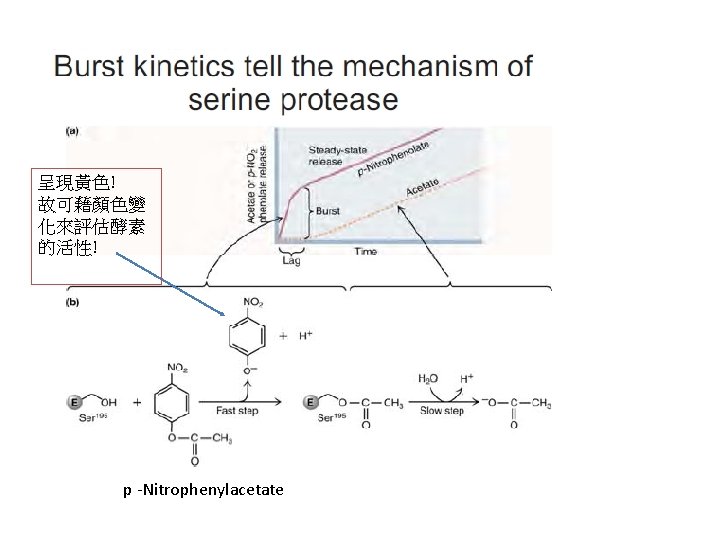
Covalent catalysis & general acid-base catalysis 詳見: “The Serine Protease Mechanism in Detail” (p. 62~p. 65)



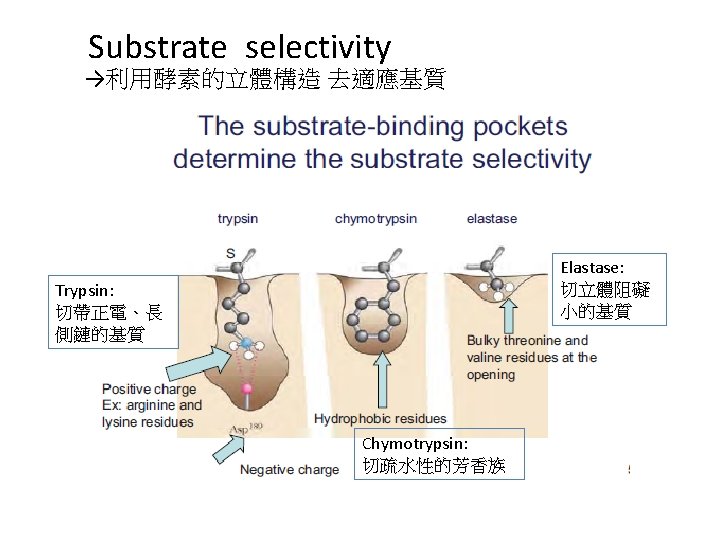
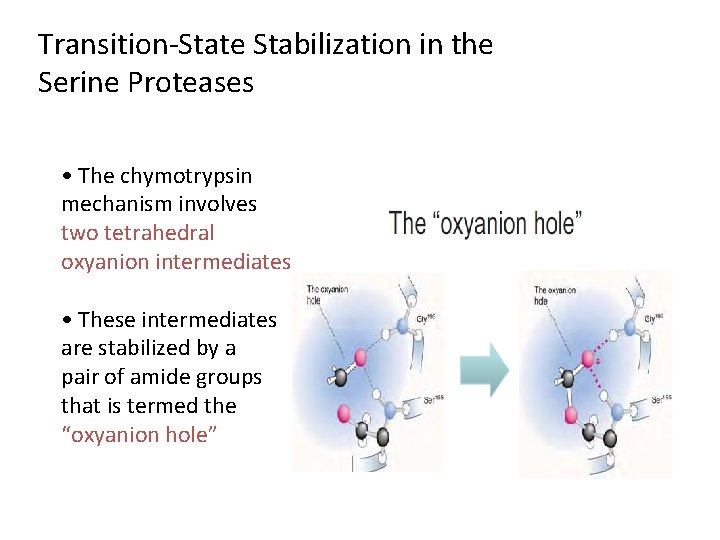
Transition-State Stabilization in the Serine Proteases • The chymotrypsin mechanism involves two tetrahedral oxyanion intermediates • These intermediates are stabilized by a pair of amide groups that is termed the “oxyanion hole”

例二: The aspartic protease: →general acid-base catalysis, LBHB • All involve two Asp residues at the active site • These two Asp residues work together as general acid-base catalysts • Most aspartic proteases have a tertiary structure consisting of two lobes (N-terminal and C-terminal) with approximate two-fold symmetry

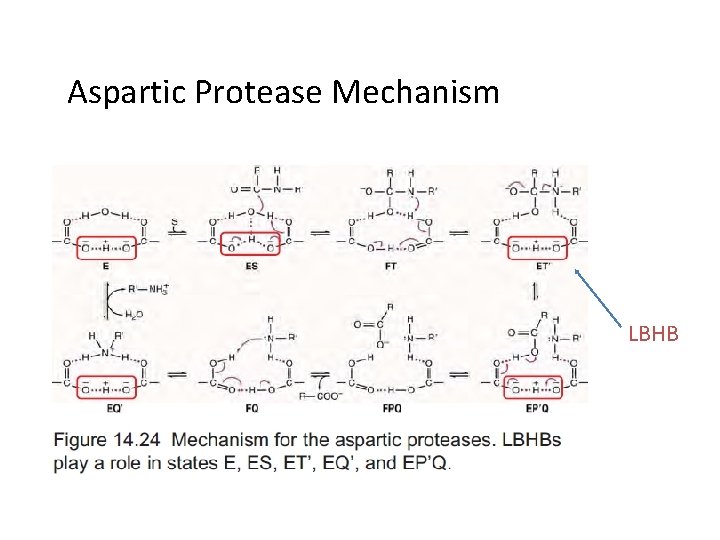
Aspartic Protease Mechanism LBHB

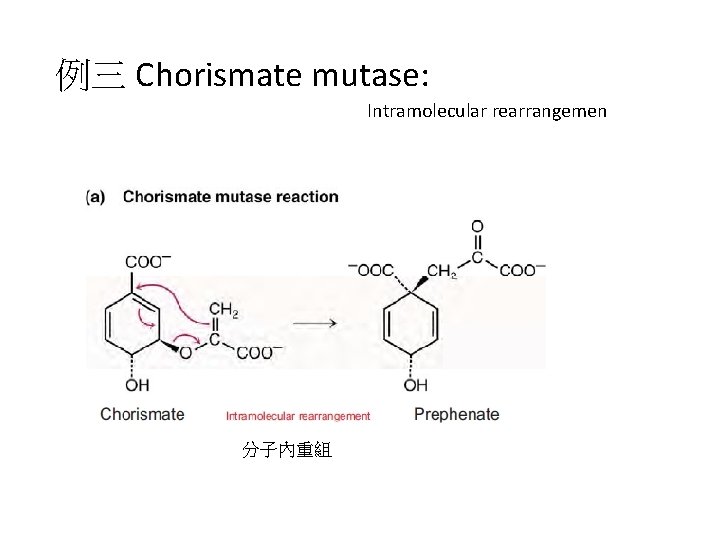
例三 Chorismate mutase: Intramolecular rearrangemen 分子內重組

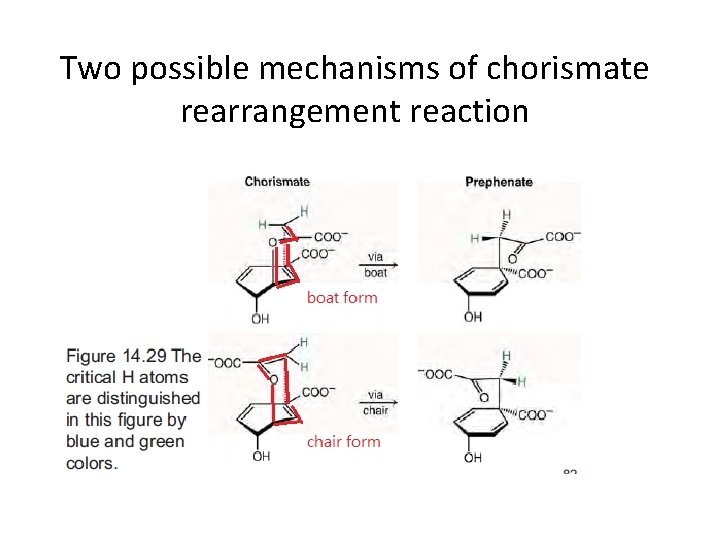
Two possible mechanisms of chorismate rearrangement reaction

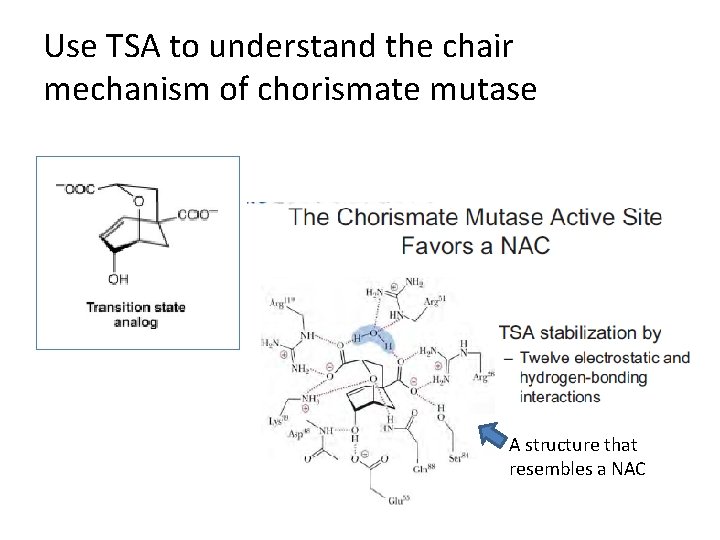
Use TSA to understand the chair mechanism of chorismate mutase A structure that resembles a NAC

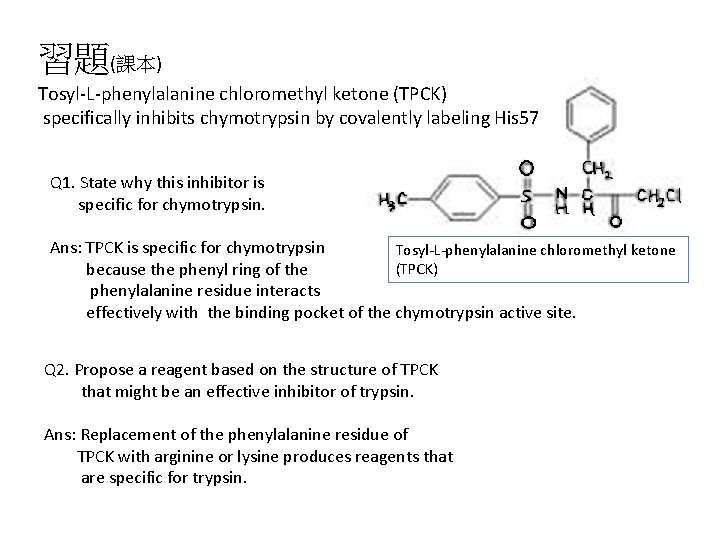
習題(課本) Tosyl-L-phenylalanine chloromethyl ketone (TPCK) specifically inhibits chymotrypsin by covalently labeling His 57 Q 1. State why this inhibitor is specific for chymotrypsin. Ans: TPCK is specific for chymotrypsin Tosyl-L-phenylalanine chloromethyl ketone (TPCK) because the phenyl ring of the phenylalanine residue interacts effectively with the binding pocket of the chymotrypsin active site. Q 2. Propose a reagent based on the structure of TPCK that might be an effective inhibitor of trypsin. Ans: Replacement of the phenylalanine residue of TPCK with arginine or lysine produces reagents that are specific for trypsin.

serine 195