Zumdahl De Coste World of CHEMISTRY Chapter 3





























- Slides: 49

Zumdahl De. Coste World of CHEMISTRY

Chapter 3 Chemical Foundations: Elements, Atoms, and Ions Copyright© by Houghton Mifflin Company. All rights reserved.

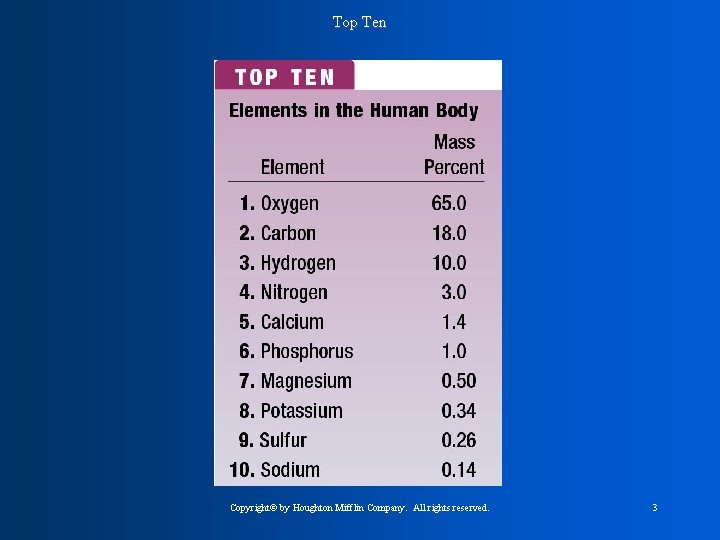
Top Ten Copyright© by Houghton Mifflin Company. All rights reserved. 3

* An ELEMENT is the simplest form of a substance that can not be broken down. * A COMPOUND is two or more elements bound together. **Presently there are 115 elements, 88 of which occur naturally.

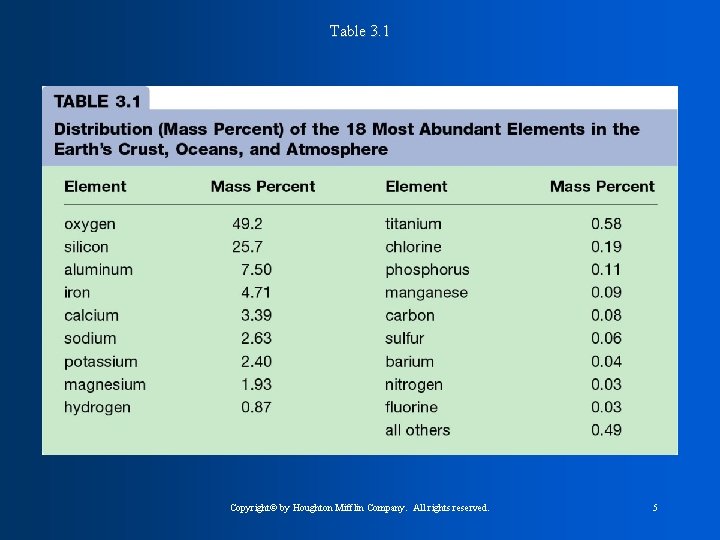
Table 3. 1 Copyright© by Houghton Mifflin Company. All rights reserved. 5

The 9 elements that account for 98% of the earths total mass (table 3. 1) * Oxygen * Silicon * aluminum * Iron * calcium * sodium * potassium * magnesium * hydrogen

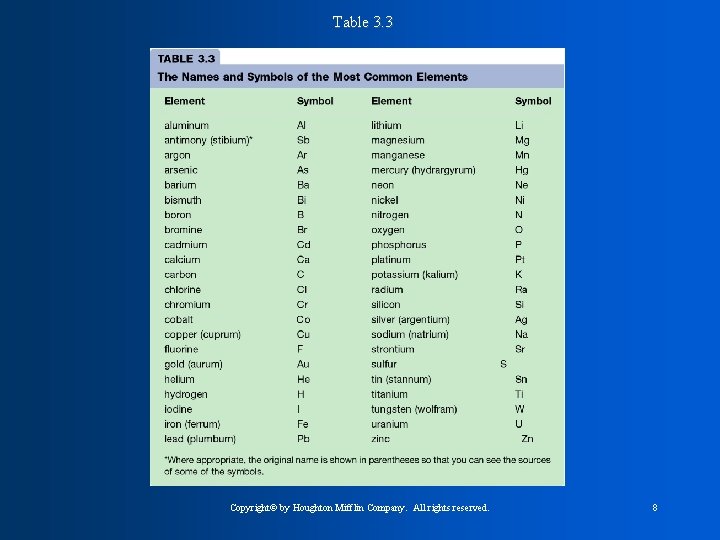
* Names of the elements originated mainly from GREEK. LATIN, and GERMAN. * We abbreviate the elements to simplify. -Always us a CAPITAL letter for the first letter in element. -use lower case if there is a second letter. Ex: Hydrogen = H Gold = Au (Aurum is latin for shiny)

Table 3. 3 Copyright© by Houghton Mifflin Company. All rights reserved. 8

Dalton's Atomic Theory 1. Elements are made of tiny particles called atoms 2. All atoms of a given element are identical 3. Atoms of a given element are different from those of any other element. 4. Atoms of one can combine with atoms of another to form compounds 5. Atoms are indivisible in chemical processes, they are not created or destroyed.

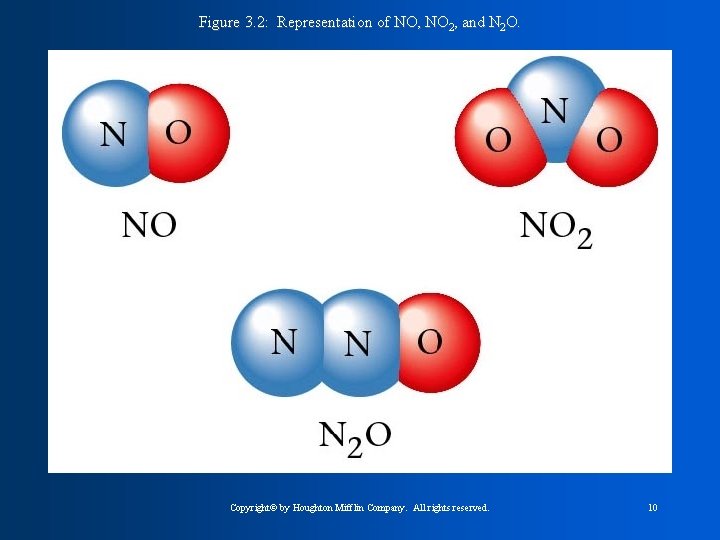
Figure 3. 2: Representation of NO, NO 2, and N 2 O. Copyright© by Houghton Mifflin Company. All rights reserved. 10


Rules for Writing Formulas 1. Each atom present is represented by its element symbol. 2. The number of each type of atom is indicated by a subscript written to the right of the element symbol. 3. When only one atom of a given type is present, the subscript 1 is not written. Copyright© by Houghton Mifflin Company. All rights reserved. 12

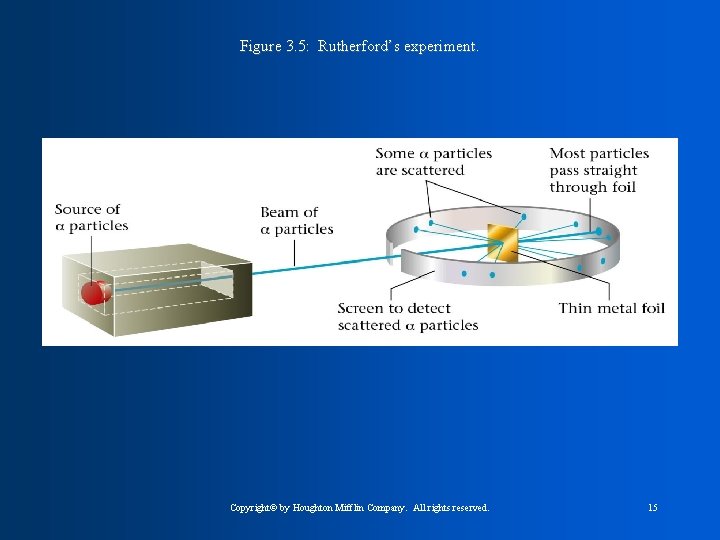
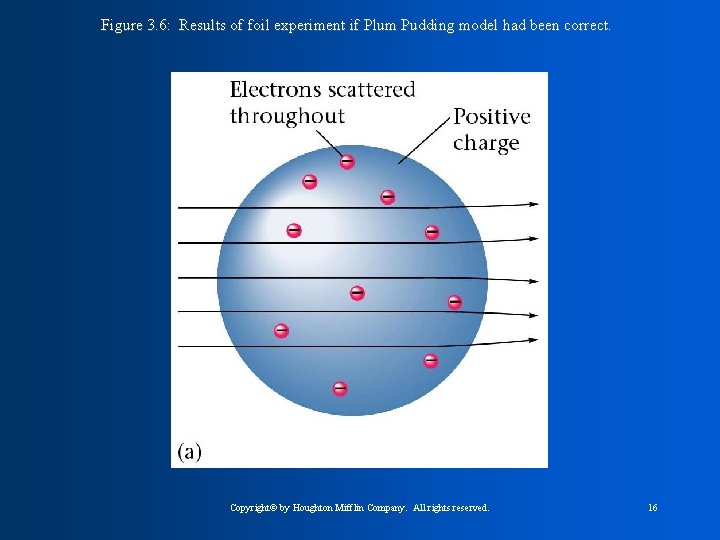
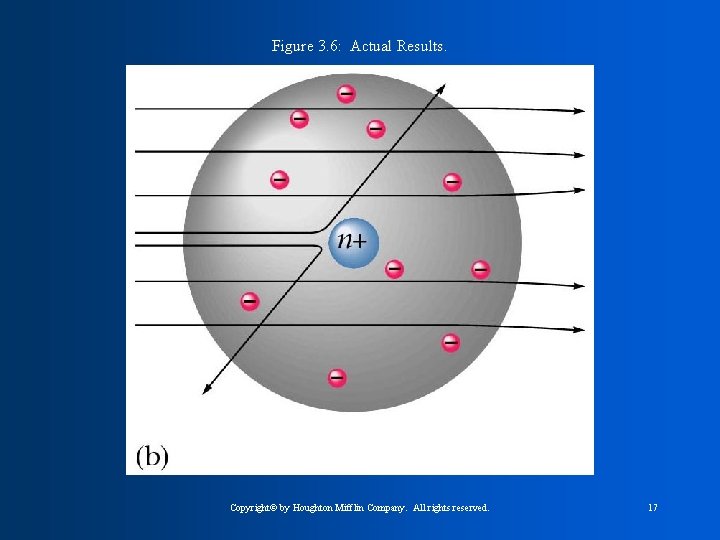
J. J Thomson concluded that all atoms contain negative particles called electrons and must then also contain positive particles called protons. William Thompson was for the PLUM-PUDDING model. He said the atom was like plum pudding ( bowl of pudding with things floating in it). Atom has pudding of positive and negative charges scattered around to counter balance. Rutherford found that atoms must have a nucleus with a dense positive charge. He concluded that the nucleus contains protons, which are positive charge, and also neutrons which have no charge.

Figure 3. 3: Plum Pudding model of an atom. Copyright© by Houghton Mifflin Company. All rights reserved. 14

Figure 3. 5: Rutherford’s experiment. Copyright© by Houghton Mifflin Company. All rights reserved. 15

Figure 3. 6: Results of foil experiment if Plum Pudding model had been correct. Copyright© by Houghton Mifflin Company. All rights reserved. 16

Figure 3. 6: Actual Results. Copyright© by Houghton Mifflin Company. All rights reserved. 17

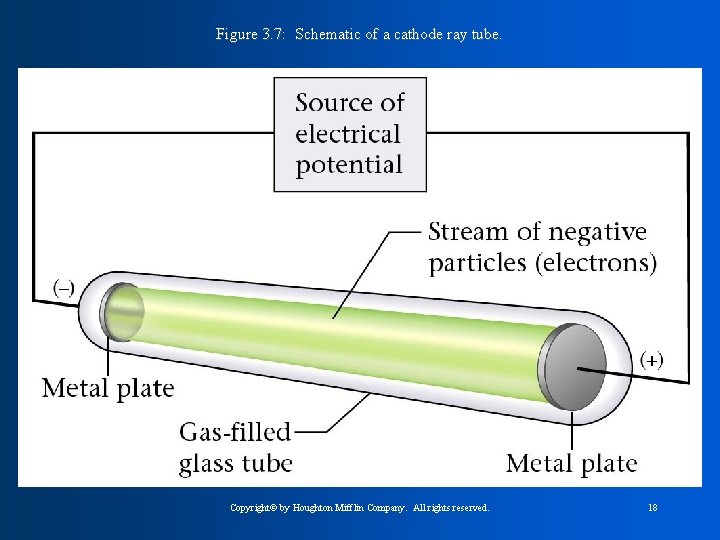
Figure 3. 7: Schematic of a cathode ray tube. Copyright© by Houghton Mifflin Company. All rights reserved. 18

Atoms can be divided into 3 subatomic Particles: 1. Protons 2. Neutrons 3. Electrons Particle Symbol Charge Mass location Proton P + 1 nucleus Neutron N 0 1 Nucleus Electron E - 1/1800 orbits

Figure 3. 9: A nuclear atom viewed in cross section. Copyright© by Houghton Mifflin Company. All rights reserved. 20

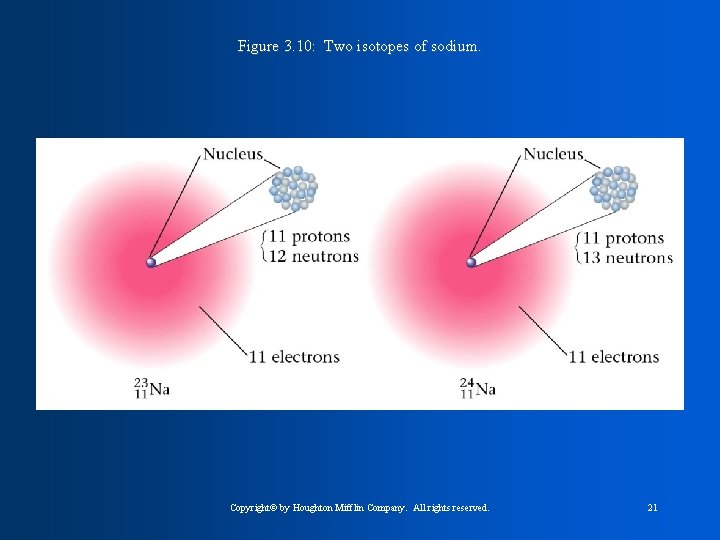
Figure 3. 10: Two isotopes of sodium. Copyright© by Houghton Mifflin Company. All rights reserved. 21

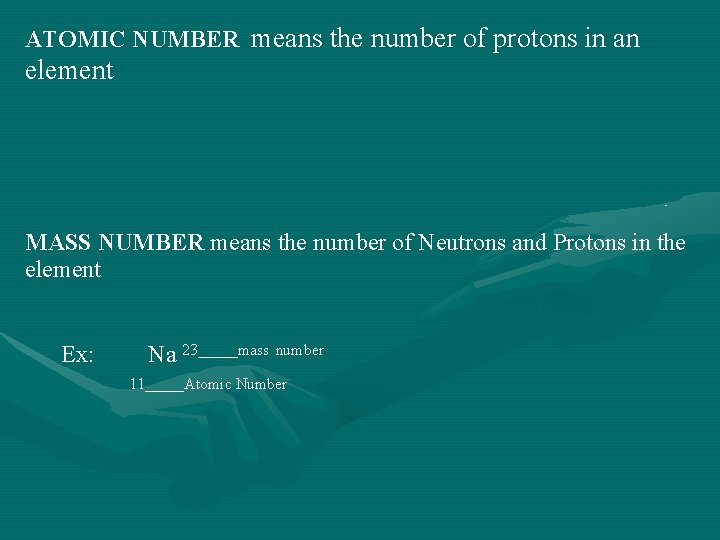
ATOMIC NUMBER means the number of protons in an element MASS NUMBER means the number of Neutrons and Protons in the element Ex: Na 23_____mass number 11_____Atomic Number

Isotopes are atoms of the same element that have different numbers of neutrons. Isotopes have the same atomic number, but different mass number

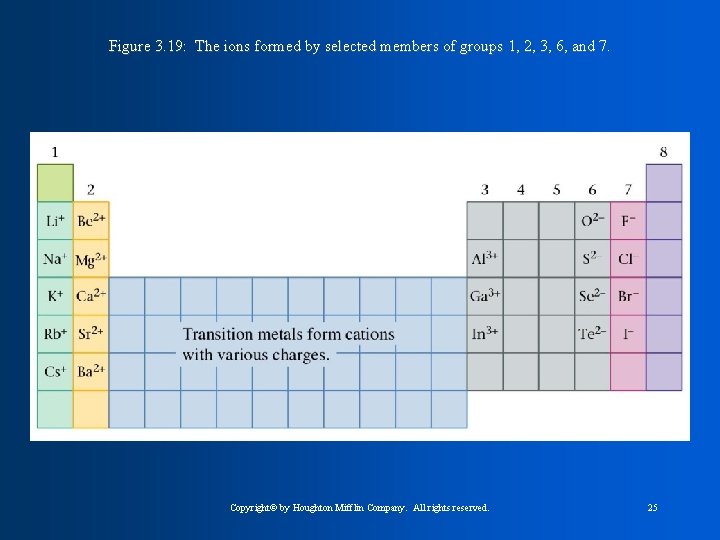
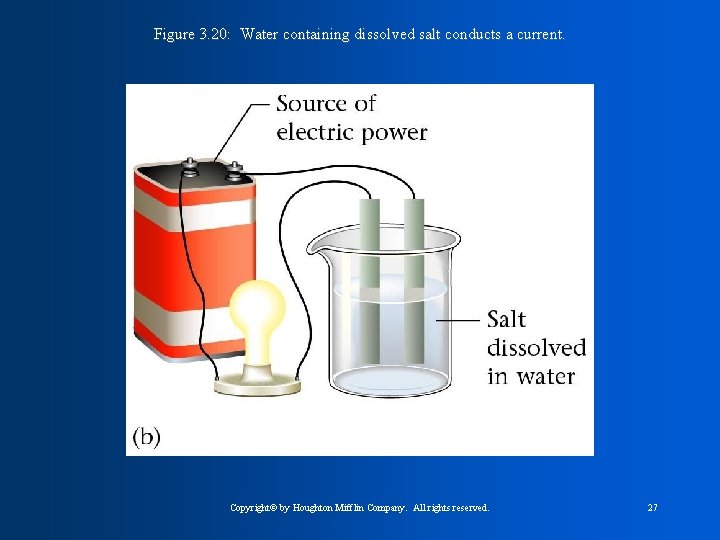
-Ion: An element, or atom, with a charge. Either positive or negative. Elements receive charges by the movement or transfer of electrons. **-If electrons are lost, they become positive. This is called a CATION. Ex. Mg 2+ -**if electrons are gained, they become negative. This is called an ANION. Ex. Cl -1 -Charges are represented with superscript numbers. Ionic Compounds contain a metal and non-metal. -Net Charges must always equal zero.

Figure 3. 19: The ions formed by selected members of groups 1, 2, 3, 6, and 7. Copyright© by Houghton Mifflin Company. All rights reserved. 25

Figure 3. 20: Pure water does not conduct a current. Copyright© by Houghton Mifflin Company. All rights reserved. 26

Figure 3. 20: Water containing dissolved salt conducts a current. Copyright© by Houghton Mifflin Company. All rights reserved. 27

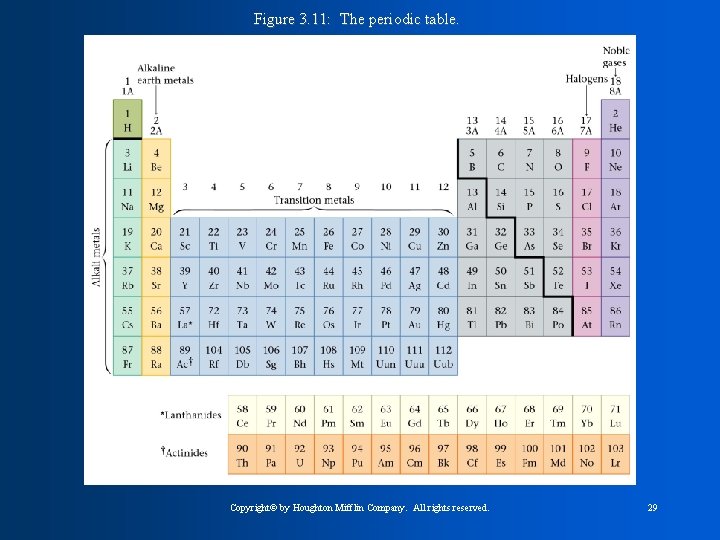
The known elements were organized according to their atomic masses in 1871 by MENDELEEV. The modern version of his table is called THE PERIODIC TABLE OF CONTENTS The Horizontal rows are called PERIODS The vertical columns are called GROUPS The Main Group Elements are in groups 1, 2, 13, 14, 15, 16, 17, and 18 Transition Metals are elements in groups 3 -12

Figure 3. 11: The periodic table. Copyright© by Houghton Mifflin Company. All rights reserved. 29

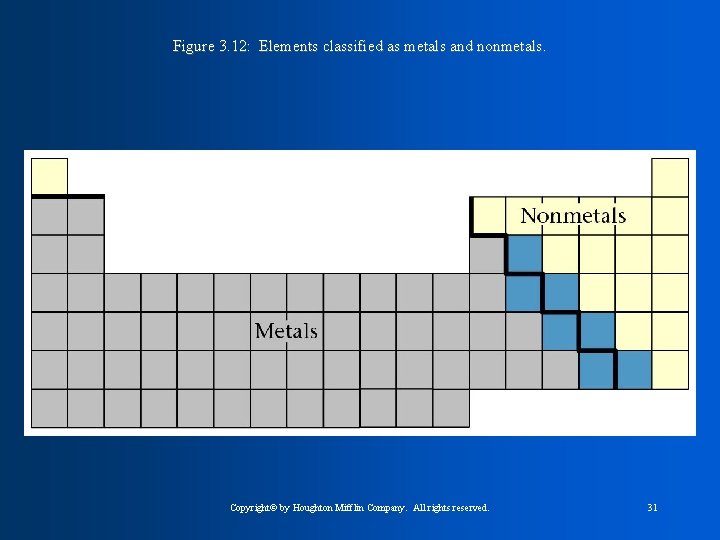
All Elements can be divided into three main categories: 1. Metals 2. Nonmetals 3. Metalloids

Figure 3. 12: Elements classified as metals and nonmetals. Copyright© by Houghton Mifflin Company. All rights reserved. 31

The upper right hand corner of the Table is where the NONMETALS are found, including Hydrogen Properties of NONMETALS: 1. poor conductors of HEAT and ELECTRICITY 2. Do Not have a shiny appearance or reflect light well 3. are brittle in the solid form

The Metalloids are the 6 elements: B, Si, Ge, As, Sb, and Te * sometimes called semi-metals -They separate the metals and non-metals and be found hugging the staircase line. -Properties: -1. poor conductors of heat -2. semi-conductors of electricity -3. have a dull appearance and do not reflect light as well -4. are brittle solids at 25° C.

The most important metalloid is Silicon, which is use to make computer chips

Group 1 Elements are also called ALKALI METALS Examples are: Li, Na, K, Rb, Cs, and Fr. Group 2 Elements are ALKALINE EARTH METALS Examples are: Be, Mg, Ca, Sr, Ba, and Ra

Group 17 Elements are called the HALOGENS Examples are F, Cl, Br, I, A Group 18 elements are called NOBLE GASES (or inert gases, or rare gases) Examples are : He, Ne, Ar, Kr, Xe, Rn

ALKALI METALS - are very Reactive, which means they readily combine with other elements. ** React with chlorine gas to form a metal chloride ** React with water to form hydrogen gas

ALKALINE EARTH METALS - they are reactive, but not as reactive as the alkali metals. ** react with chlorine gas to form a metal chloride ** react with water to form hydrogen gas (sodium reacts with water at room temperature, but magnesium won’t react with water unless the water is boiling. )

Alkali Metals and Alkaline Earth Metal have been used in everyday items such as: *Lithium was used to treat manic depression -7 -UP used to contain lithium *Magnesium is used in disposable flash bulbs *Calcium can be found in limestone, chalk, marble, and coral.

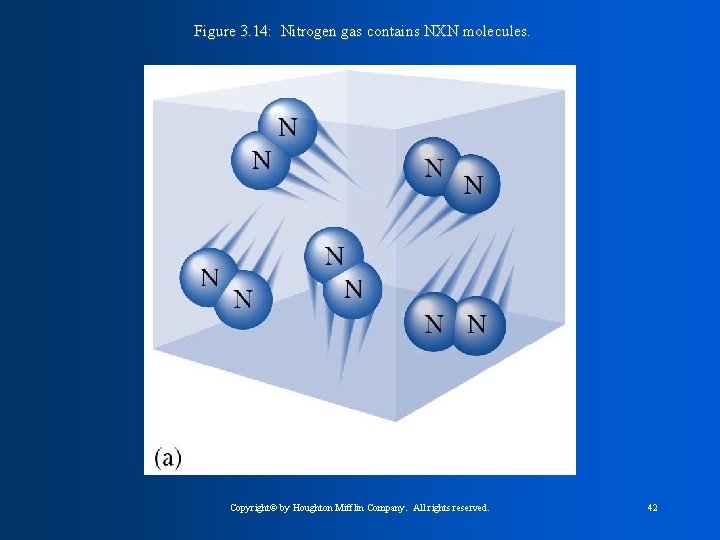
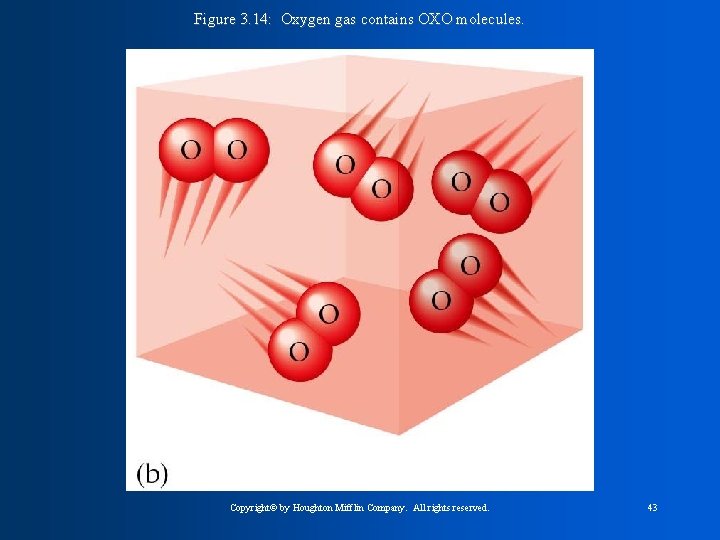
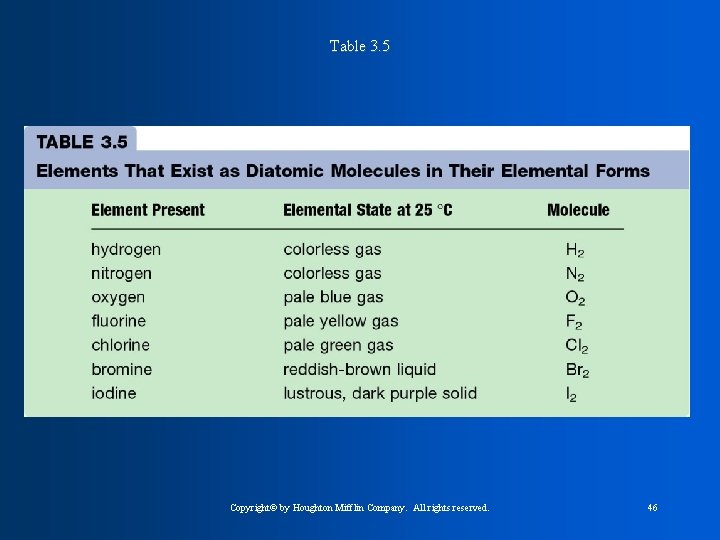
The Halogens * are non-metals and exist as diatomic molecules ( a molecule that contains 2 atoms. - Homonuclear diatomic molecules are the same element F 2, Cl 2, Br 2, and I 2 -Heteronuclear diatomic molecules are different CO, NO, elements:

Halogens are reactive and react with Hydrogen gas to form compounds with the formula (HX) Ex. -HF is used to etch glass (lightbulbs, TV tubes) -HCl is used to digest food

Figure 3. 14: Nitrogen gas contains NXN molecules. Copyright© by Houghton Mifflin Company. All rights reserved. 42

Figure 3. 14: Oxygen gas contains OXO molecules. Copyright© by Houghton Mifflin Company. All rights reserved. 43

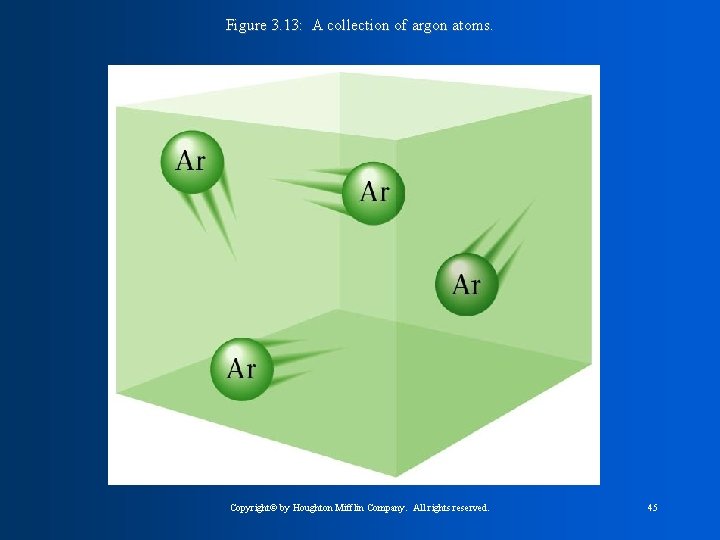
Noble Gases: - They are all colorless gases at 25°C. - They are unreactive (very stable) Ex: Neon, used in neon signs (zapped by electricity)

Figure 3. 13: A collection of argon atoms. Copyright© by Houghton Mifflin Company. All rights reserved. 45

Table 3. 5 Copyright© by Houghton Mifflin Company. All rights reserved. 46

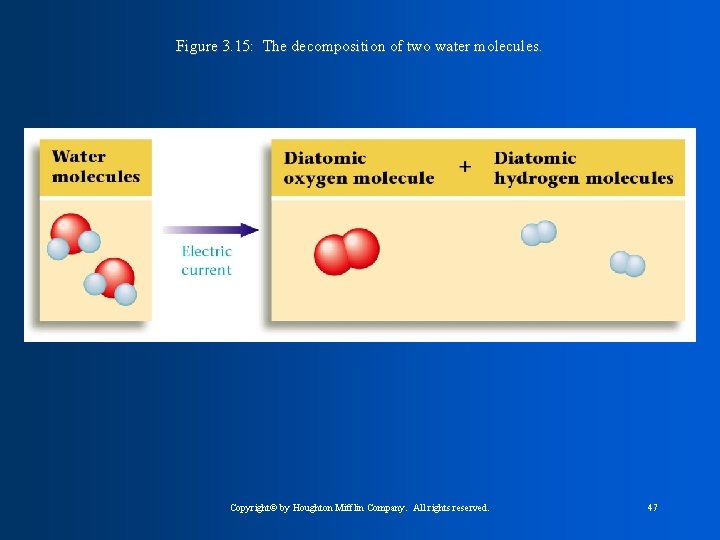
Figure 3. 15: The decomposition of two water molecules. Copyright© by Houghton Mifflin Company. All rights reserved. 47

Figure 3. 17: Spherical atoms packed closely together. Copyright© by Houghton Mifflin Company. All rights reserved. 48

Terms to Know: Melting Point: goes from solid to liquid Freezing point: goes from liquid to solid Evaporation: Condensation: goes from liquid to gas goes from gas to liquid Sublimation: goes from solid to gas Deposition: goes from gas to solid
 Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes C / lambda
C / lambda Azimuthal quantum number
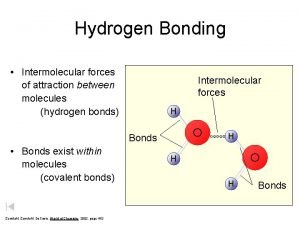
Azimuthal quantum number H-bonding intermolecular forces
H-bonding intermolecular forces Zumdahl chemistry
Zumdahl chemistry Zumdahl chapter 4
Zumdahl chapter 4 Zumdahl chapter 12
Zumdahl chapter 12 Busqueda de coste uniforme
Busqueda de coste uniforme Coste explícito
Coste explícito Coste marginal
Coste marginal Activos financieros a coste
Activos financieros a coste Il mare di baffin lambisce le coste
Il mare di baffin lambisce le coste Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Ap world history chapter 25 africa and the atlantic world
Ap world history chapter 25 africa and the atlantic world Old vs new world monkeys
Old vs new world monkeys Pumpkin new world or old world
Pumpkin new world or old world Real world vs digital world
Real world vs digital world Plato's forms
Plato's forms The changing world output and world trade picture
The changing world output and world trade picture Dangerous world tour
Dangerous world tour What plots the maps of the slum children
What plots the maps of the slum children On sour cream walls donations figure of speech
On sour cream walls donations figure of speech English 9 unit 9
English 9 unit 9 The changing world output and world trade picture
The changing world output and world trade picture Is chalk natural or manmade
Is chalk natural or manmade Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Intro to organic chemistry
Intro to organic chemistry Chapter 9 review stoichiometry
Chapter 9 review stoichiometry Love formula
Love formula Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 review acids and bases
Chapter 14 review acids and bases Chapter 13 ions in aqueous solutions
Chapter 13 ions in aqueous solutions Modern chemistry chapter 12 review answers
Modern chemistry chapter 12 review answers Avogadro's law relationship
Avogadro's law relationship Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 assessment the mole answer key
Chapter 10 assessment the mole answer key Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Chapter 7 organic chemistry
Chapter 7 organic chemistry Chapter 24: nuclear chemistry answer key
Chapter 24: nuclear chemistry answer key Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Chapter 12 basics of chemistry cosmetology
Chapter 12 basics of chemistry cosmetology Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Reaction of grignard reagent with acid chloride
Reaction of grignard reagent with acid chloride Modern chemistry chapter 4
Modern chemistry chapter 4 Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers