ZOONOTIC DISEASES Dr Aslesh OP MBBS MD Community

ZOONOTIC DISEASES Dr Aslesh OP MBBS, MD Community Medicine (AIIMS) Assistant professor Pariyaram medical college

DEFINITION • • • Zoonoses are infections which are naturally transmitted between vertebrate animals and people (WHO 1959) Derived from the Greek ZOON (animals) and NOSES (diseases) People, animals, birds, arthropods and the inanimate environment are all involved in cycles of zoonotic infection

ZOONOSES DEFINITIONS Anthropozoonoses: Zoonoses where the main reservoir of infection is non-human vertebrate animals Most zoonoses are of this type e. g. bovine tuberculosis, rabies, leptospirosis Zooanthroponoses: Diseases that mainly affect people, which may be transmitted to animals, which then act as temporary reservoirs of infection Examples are Mycobacterium tuberculosis in dogs, infectious hepatitis in apes, H 1 N 1 pandemic influenza in pigs Slide 1
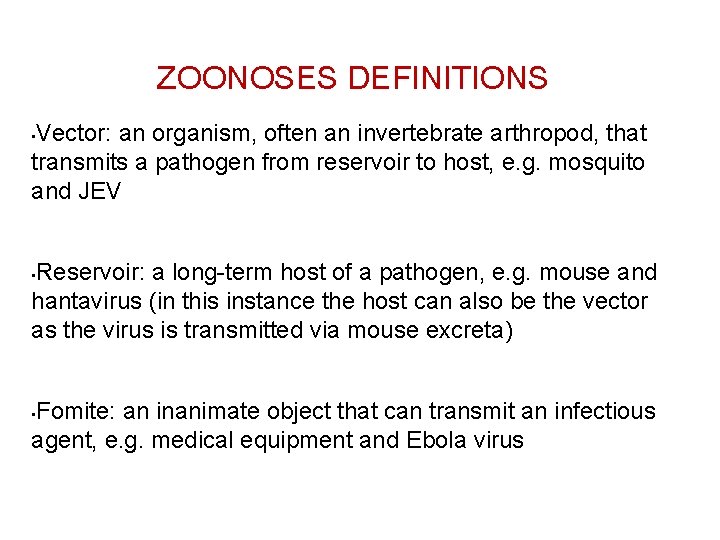
ZOONOSES DEFINITIONS Vector: an organism, often an invertebrate arthropod, that transmits a pathogen from reservoir to host, e. g. mosquito and JEV • Reservoir: a long-term host of a pathogen, e. g. mouse and hantavirus (in this instance the host can also be the vector as the virus is transmitted via mouse excreta) • Fomite: an inanimate object that can transmit an infectious agent, e. g. medical equipment and Ebola virus •
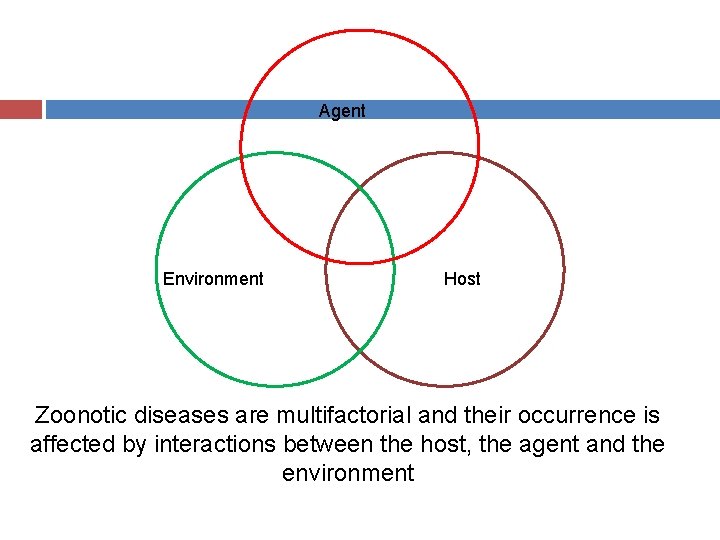
Agent Environment Host Zoonotic diseases are multifactorial and their occurrence is affected by interactions between the host, the agent and the environment

ZOONOSES Approximately 1500 infectious diseases are recognized in humans Of these 20% (300) are due to zoonoses however 75% of recently emerging infectious diseases (EID) have been caused by zoonotic pathogens Slide 05

EMERGING DISEASES WHO definition “An emerging zoonosis is a zoonosis that is newly recognised or newly evolved, or that has occurred previously but shows an increase in incidence or expansion in geographical, host or vector range”

REASONS FOR DISEASE EMERGENCE 1. Factors explaining emergence are complex, e. g. genetic drift and shift (influenza) and modification of the immunological status of populations (change in susceptibility of populations; vaccination) 2. Social and ecological conditions influencing population growth and movement, food habits and the environment may be more important 3. Influence of changing environment on reservoirs, vectors and victim species 4. Population expansion and urbanisation

REASONS FOR ZOONOSIS EMERGENCE 5. Increasing contact between human populations, wild and domestic animals expose people to zoonotic agents by direct or indirect contact 5. Growing population of drug and alcohol impaired and immunosuppressed people worldwide are at higher risk of zoonoses 7. Many of the common, life threatening infections associated with HIV are zoonoses Slide 09

IMPACT OF ZOONOTIC DISEASES 13 insert your company logo Human health (death and disability) Animal health (death and disability) Economic losses due to livestock culling, deaths and decreased production Cost of control programs Trade implications Indirect effects on people due to psychological trauma (loss of pets, culling, loss of valuable animals) and impact on cultural traditions, etc.

CLASSIFICATION OF ZOONOSES Type of infectious agent (bacteria, fungus, virus, parasite) Mode of transmission Type of reservoir host
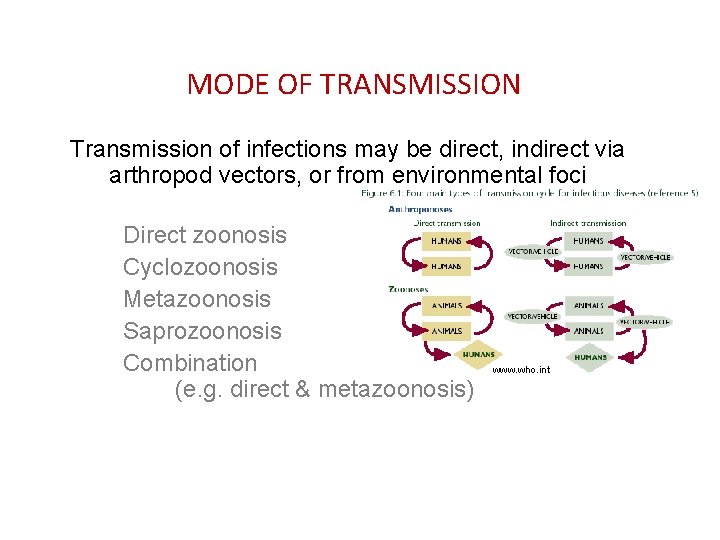
MODE OF TRANSMISSION Transmission of infections may be direct, indirect via arthropod vectors, or from environmental foci Direct zoonosis Cyclozoonosis Metazoonosis Saprozoonosis Combination (e. g. direct & metazoonosis) www. who. int
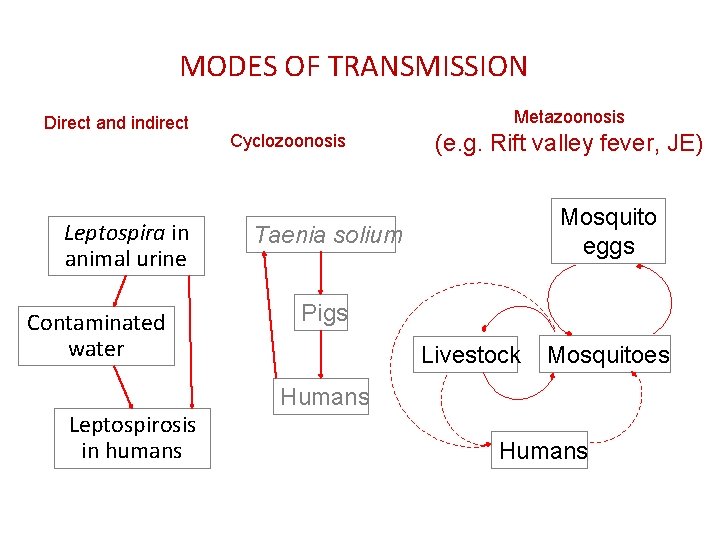
MODES OF TRANSMISSION Direct and indirect Leptospira in animal urine Contaminated water Leptospirosis in humans Metazoonosis Cyclozoonosis (e. g. Rift valley fever, JE) Mosquito eggs Taenia solium Pigs Livestock Mosquitoes Humans

SAPROZOONOSIS Diseases of vertebrate animals which can affect people, the infectious agents of which are either capable of replicating in inanimate sites, or require an inanimate environment for the development of an infectious stage of their life cycle eg. histoplasmosis, Toxocara canis, certain food-borne diseases

SUMMARY OF SELECTED ZOONOSES BACTERIAL Anthrax Brucellosis Plague Leptospirosis

LEPTOSPIROSIS • Disease described in dogs in 1850 • Weil’s disease described in people in 1880 • Organism first isolated in Japan in 1914 - now 7 pathogenic species & over 200 serovars recognised • By 1940 leptospirosis established as a major animal and public health problem • Occupational disease • Associated with natural disasters e. g. floods Slide 29
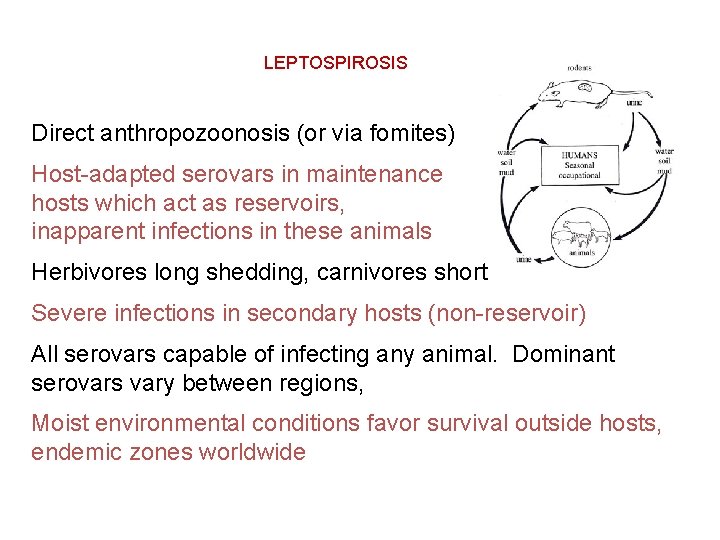
LEPTOSPIROSIS Direct anthropozoonosis (or via fomites) Host-adapted serovars in maintenance hosts which act as reservoirs, inapparent infections in these animals Herbivores long shedding, carnivores short Severe infections in secondary hosts (non-reservoir) All serovars capable of infecting any animal. Dominant serovars vary between regions, Moist environmental conditions favor survival outside hosts, endemic zones worldwide

LEPTOSPIROSIS Occupational hazard: in rice-growing communities, Recreational risks: Use of ponds Natural disasters: floods

Zoonotic diseases: Session # LEPTOSPIRA AND HUMAN DISEASE • Contact with infected urine or contaminated water, seasonal spring/summer in cooler climates • Entry via intact mucous membranes, aerosols or skin abrasions, occupational disease • Anicteric (without jaundice) disease is common form in Australia, vague symptoms, flu-like, fever, headache, myalgia • Icteric disease very severe, more common in Asia, rodent hosts of serovars • Person-person transmission rare, dead-end hosts Slide 32

CONTROL OF LEPTOSPIROSIS Chemoprophylaxis; doxycycline 100 mg weekly Protective clothing, e. g. boots, eye protection, gloves in abattoirs Avoid contact with animal urine Control of wild reservoirs e. g. Rodents Immersion in natural water source to be avoided

ANTHRAX • Bacillus anthracis, Gram-positive sporing rod • Worldwide, Russia, Africa, South America • ‘Hot spots’ in warm humid areas where natural cycles exist • Enzootic in south india • All mammals susceptible but pigs, dogs and cats relatively resistant • Birds can disseminate spores, chickens resistant, some birds susceptible Slide 19

ANTHRAX Organisms enter via Skin (cutaneous anthrax) Lungs (pulmonary anthrax, woolsorter’s disease) Gastrointestinal tract Clinical signs include Eschar in humans (black necrotic skin lesion) Sudden death in ungulates, blood from the nose and other body orifices; septicemia in humans

ANTHRAX http: //www. extension. org/pages/Anthrax

ANTHRAX TRANSMISSION AND HUMAN DISEASE • Animal by-products, wool, hides, bone meal, meat, etc. involved in spore transmission • Cutaneous infections most common, inhalation, intestinal, person-to-person rare • 1 -7 d incubation, spores germinate, papules, vesicles, edema, fatal septicemia • Agricultural workers, vets, rural communities, travellers etc. • Bioterrorism agent – US postal workers affected

Prevention and control measures Surveillance and reporting Safe disposal of carcasses collection in double layered plastic sheets Burning and safe disposal of ashes Dust control measures in hazardous area Quarantine- 20 days after last cases Vaccination 3 subcutaneous doses 2 weeks apart and subsequent doses in 6, 12, 18 months Annual booster injection

BRUCELLOSIS • Gram negative rod shaped, nonmotile, non spore forming intracellular coccobacilli • B. melitensis - goats, sheep (cattle, camels, dogs, people). Asia, Africa, Mediterranean and South America • B. abortus – bovines, (dogs, people). Worldwide • B. suis – pigs (people). South America, Southeast Asia, United States, Australia • B. canis - domestic and wild carnivores (people). Many countries worldwide • Primary hosts susceptible, disease in other hosts varies in severity
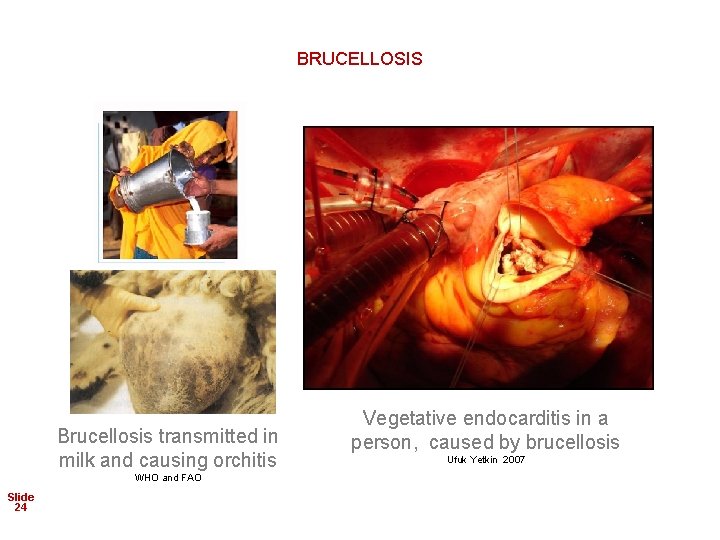
BRUCELLOSIS Brucellosis transmitted in milk and causing orchitis WHO and FAO Slide 24 Vegetative endocarditis in a person, caused by brucellosis Ufuk Yetkin 2007
BRUCELLOSIS • Mode of transmission • Contact infection • Food borne • Airborne • Incubation period: 1 -3 weeks but as long as 6 months Slide 25

BRUCELLOSIS • Symptoms and signs • Flu-like symptoms in humans, including muscle and joint pains, fever, cough, (rarely endocarditis) • Hepatosplenomegaly • Leucopenia with relative lymphocytosis • Diagnosis: isolation by culture • Treatment- Tetracycline (500 mg 6 th hourly for 3 weeks) Slide 25

CONTROL OF BRUCELLOSIS IN ANIMALS • Surveillance, quarantine, movement control • Test and slaughter • Serological testing - card test, Rose Bengal test, milk ring test, slaughter positives • Segregation at parturition, sanitation • Vaccination - S 19 (live), 45/20 (killed) • No vaccines for pigs or dogs
CONTROL OF BRUCELLOSIS IN Humans • Early diagnosis and treatment with tetracyclines • Pasturization of milk • Personal protective measures • Vaccination- B abortus strain 19 BA

PLAGUE Capture of rats in Indochina to prevent plague www. asnom. org/ en/423_peste. html

PLAGUE • Yersinia pestis, Gram-negative rod • Rodent disease transmitted by fleas (carried by 30 -40 species of rodent, 1500 types of fleas) • Complex sylvatic (wild) cycle • Rodents act as reservoir and amplifying host • Humans accidental hosts • Carnivores infected by ingestion
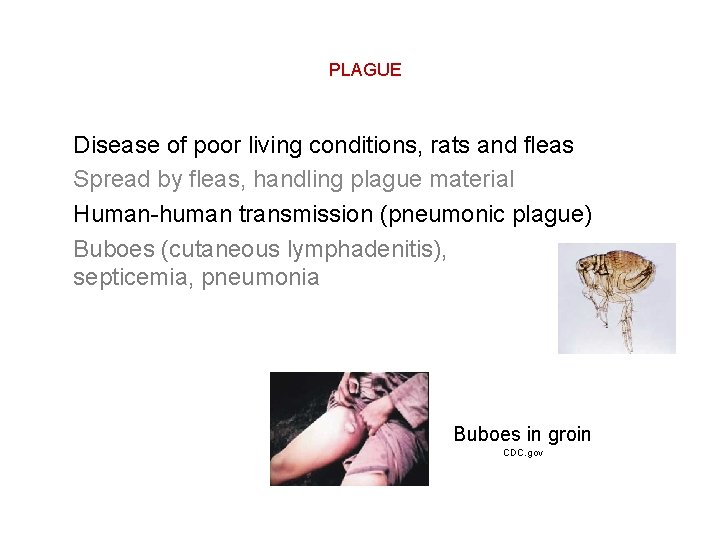
PLAGUE Disease of poor living conditions, rats and fleas Spread by fleas, handling plague material Human-human transmission (pneumonic plague) Buboes (cutaneous lymphadenitis), septicemia, pneumonia Buboes in groin CDC. gov

Prevention and control • Rodent control (cover feed, alter habitats) • Flea control in rodents and pets • Effective diagnosis, early warning, treatment and quarantine • Chemoprophylaxis • Vaccines- for prevention not for control • 2 SC doses one -2 weeks apart with 6 monthly booster

SUMMARY OF SELECTED ZOONOSES VIRAL LYSSAVIRUSES (RABIES) FLAVIVIRUSES (JAPANESE ENCEPHALITIS) INFLUENZA VIRUSES SARS HANTA VIRUS

Zoonotic diseases: Session # LYSSAVIRUSES Family Rhabdoviridae, Genus Lyssavirus 1. Classical rabies 2. Lagos bat virus 3. Mokola virus 4. Duvenhage virus 5. European bat virus 1 6. European bat virus 2 7. Pteropus Lyssavirus (Australian bat lyssavirus) Slide 34

CLASSICAL RABIES Virulent rabies spread from Europe to Asia and other regions by infected dogs; many countries worldwide Dogs most important domestic hosts, cattle and other domestic animals commonly involved Many wild reservoirs which differ between regions; principally canids (foxes, wolves, jackals) but also mongooses, skunks, raccoons, bats Some countries free by eradication e. g. UK Direct zoonosis – bites, mucosal exposure, other routes e. g. corneal transplants
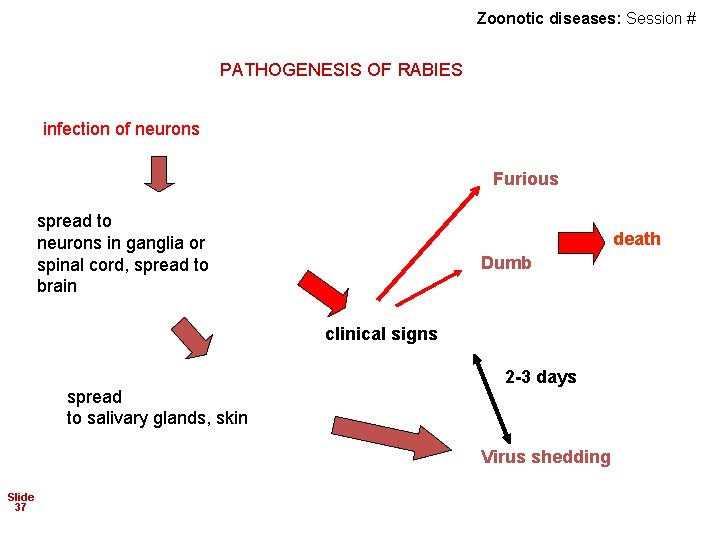
Zoonotic diseases: Session # PATHOGENESIS OF RABIES infection of neurons Furious spread to neurons in ganglia or spinal cord, spread to brain death Dumb clinical signs spread to salivary glands, skin 2 -3 days Virus shedding Slide 37

RABIES • Transmission to people mainly by bites via virus in saliva • aerosol transmission extremely rare • Aerosol transmission, found in bat saliva in zoos • Incubation 4 days – 6 years, depends on bite site • Clinical rabies invariably fatal
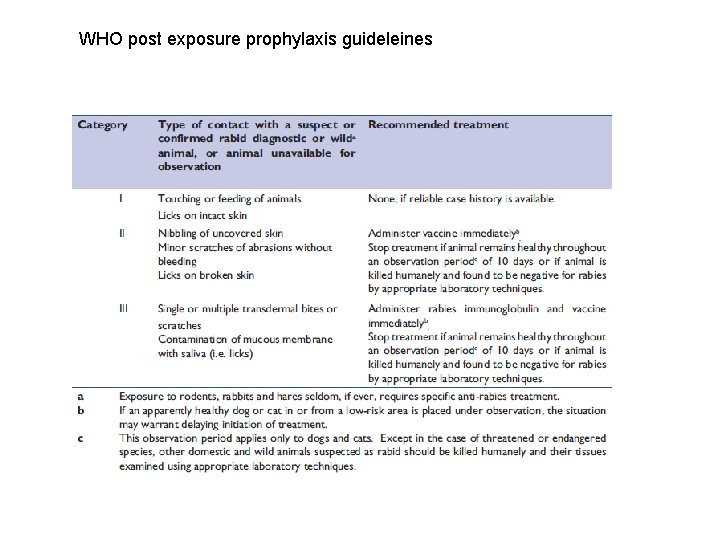
WHO post exposure prophylaxis guideleines

Management of animal bite Wound toilet In running water with soap for minimum 10 minutes Use of anteseptics Avoid suturing Antibiotic course Tetenus toxoid immunization
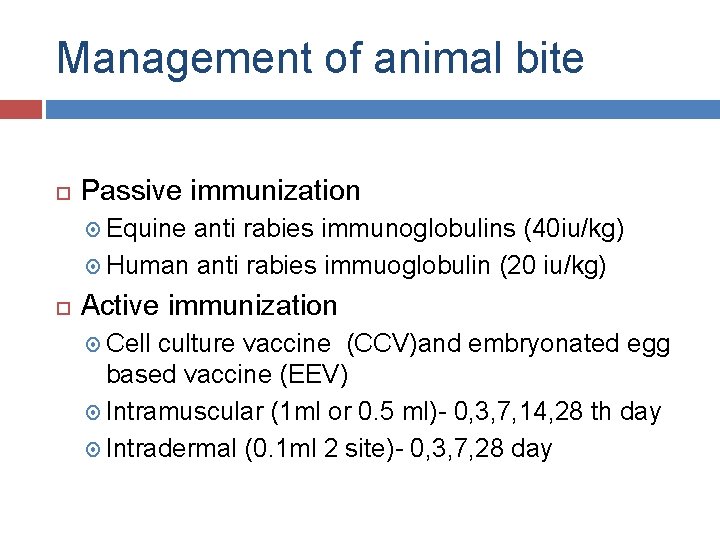
Management of animal bite Passive immunization Equine anti rabies immunoglobulins (40 iu/kg) Human anti rabies immuoglobulin (20 iu/kg) Active immunization Cell culture vaccine (CCV)and embryonated egg based vaccine (EEV) Intramuscular (1 ml or 0. 5 ml)- 0, 3, 7, 14, 28 th day Intradermal (0. 1 ml 2 site)- 0, 3, 7, 28 day

Post exposure prophylaxis of previously vaccinated person 0 and 3 rd day dose only No need of IG Pre exposure prophylaxis I ml IM in 0, 3, and 28 day Booster if antibody level falls below 0. 5 iu/ml

Japanese encephalitis Group B arbovirus (flavi virus) Transmitted by culicine mosqiuito Animal reservoir- Birds, pig Endemic in south India
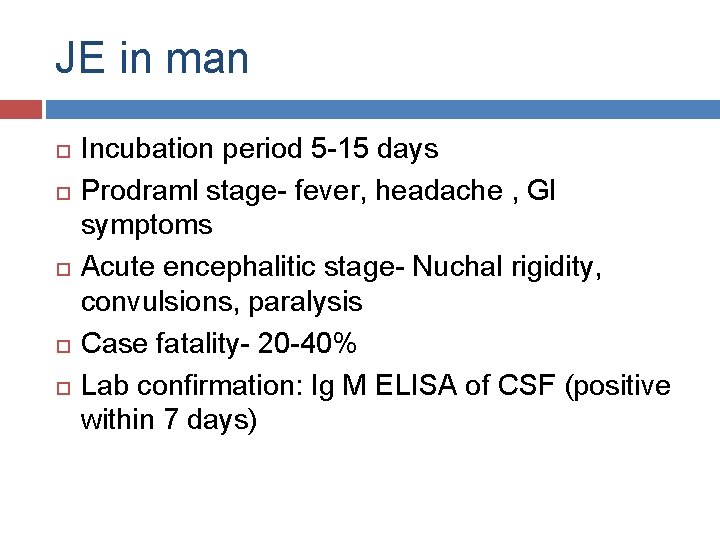
JE in man Incubation period 5 -15 days Prodraml stage- fever, headache , GI symptoms Acute encephalitic stage- Nuchal rigidity, convulsions, paralysis Case fatality- 20 -40% Lab confirmation: Ig M ELISA of CSF (positive within 7 days)
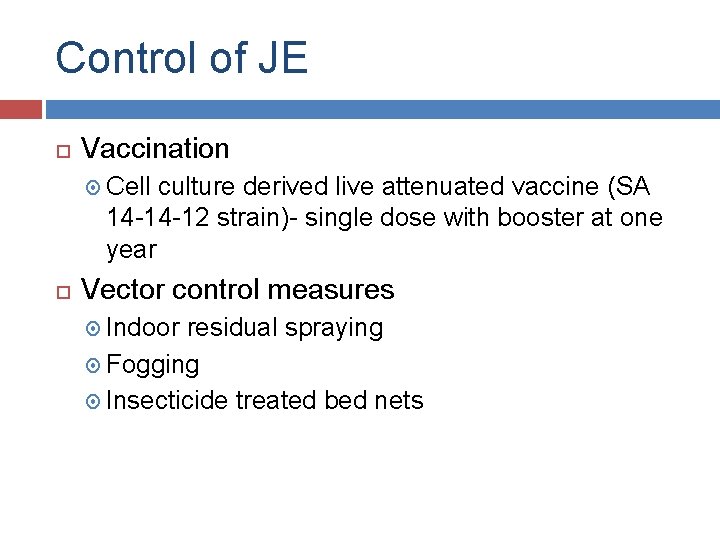
Control of JE Vaccination Cell culture derived live attenuated vaccine (SA 14 -14 -12 strain)- single dose with booster at one year Vector control measures Indoor residual spraying Fogging Insecticide treated bed nets

Kasanur forest disease Viral hemoragic fever by arbo virus (flavi virus) Seen in forest areas of south karnataka Transmitted by Tick Reservoir Primary host- rats and squirrels Amplifying host- Monkeys
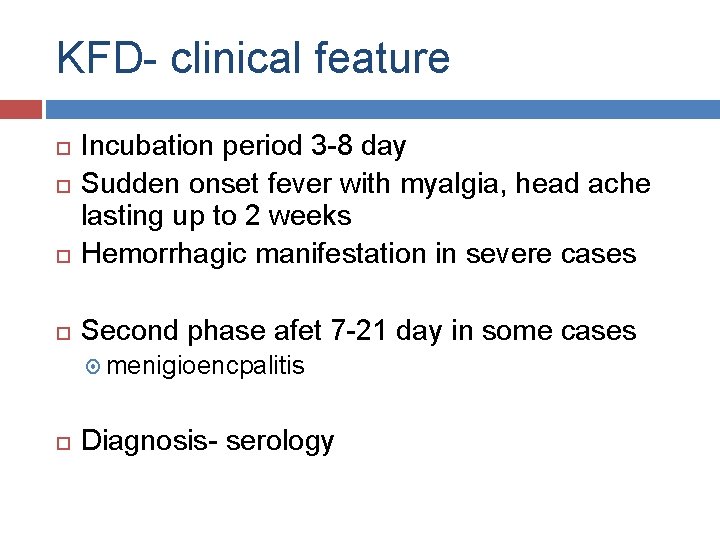
KFD- clinical feature Incubation period 3 -8 day Sudden onset fever with myalgia, head ache lasting up to 2 weeks Hemorrhagic manifestation in severe cases Second phase afet 7 -21 day in some cases menigioencpalitis Diagnosis- serology

KFD prevention and control Control of ticks Hot spot -spraying with propoxur, fention ( 50 m radius around monkey death) Vaccination- killed vaccine Personal protection- cloths repellent Checking for tick in body Avoid lying down in plain ground

SEVERE ACUTE RESPIRATORY SYNDROME Outbreak in China in November 2002 SARS coronavirus Flu-like symptoms Case fatality rate in humans of almost 10% Related to trade in wild animals, civet cats (which had been in contact with bats in the farms), market hygiene Humans infected via processing, cleaning Human-to-human transmission, 8096 cases, 774 deaths Disease control by quarantine, thermal imaging used to detect cases early in disease at airports

HANTAVIRUS First recognized in the Korean war in the 1950 s along the Hantan river New strain causing Hantavirus cardiopulmonary syndrome recognized in America in 1993 Rodent reservoir www. kuleuven. be/ rega/mvr/research. html Slide 55

HANTAVIRUS RNA viruses of the Bunyaviridae family Spread by aerosolized rodent excreta or rodent bites No human-to-human transmission Endemic in China, SE Asia, America and Europe Renal and (cardio)pulmonary syndromes Control by controlling rodent populations, do not stir up dust when cleaning rodent-infected areas

INFLUENZA VIRUSES Highly contagious acute respiratory disease, high mortality, low mortality Type A viruses are the only zoonotic ones, H 5 N 1 and H 1 N 1 are 2 newly emerged virus strains of global concern Humans, pigs, horses and avians are commonly affected, other animals rarely (cattle, mink, seals) Poultry and pigs are the main source of infection for humans, occupational exposure
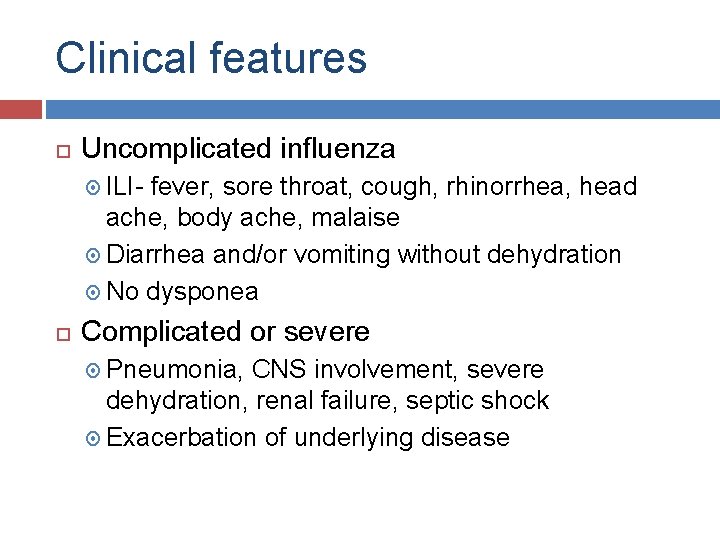
Clinical features Uncomplicated influenza ILI- fever, sore throat, cough, rhinorrhea, head ache, body ache, malaise Diarrhea and/or vomiting without dehydration No dysponea Complicated or severe Pneumonia, CNS involvement, severe dehydration, renal failure, septic shock Exacerbation of underlying disease

Infection control Hand hygiene with soap or alcohol based hand sanitizer Cover mouth and nose with tissue or cloth while coughing Face mask to be used if ill Health care professionals to use while carrying out aerosol generation procedures N 95 or FFP 2 face masks Eye protection, gown and gloves Isolation 7 days after onset of disease symptoms Prolonged isolation in immunocompromised cases 24 hours after resolution of fever and respiratory

Influenza pandemic vaccine Inactivated vaccine Monovalent containing A/california/7/2009(H 1 N 1)V like strian Single dose 0. 5 ml IM Effective after 14 days Can still get infuenza due to other strain Live attenuated Nasal spray May cause runny nose, cough , sorethroat

Whom to vaccinate Following groups to be vaccinated according to this priority order 1. 2. 3. 4. 5. 6. 7. Health care workers Pregnant women Indiviadual >6 months with chronic medical conditions Healthy young adults 15 -49 Health children Health adults between 49 -65 Health adults more than 65

Avian flu H 5 N 1 Affects birds rarely pigs and humans Potential to adapt to a strain contagious among humans threat for a pandemic New extremely severe strain circulating since 1997 Lab confirmed cases in 15 countries 25 -50% mortality in humans Preventive and control measures Hygienic handling of poultry Hand washing Cooking poultry more than 70 degrees Culling
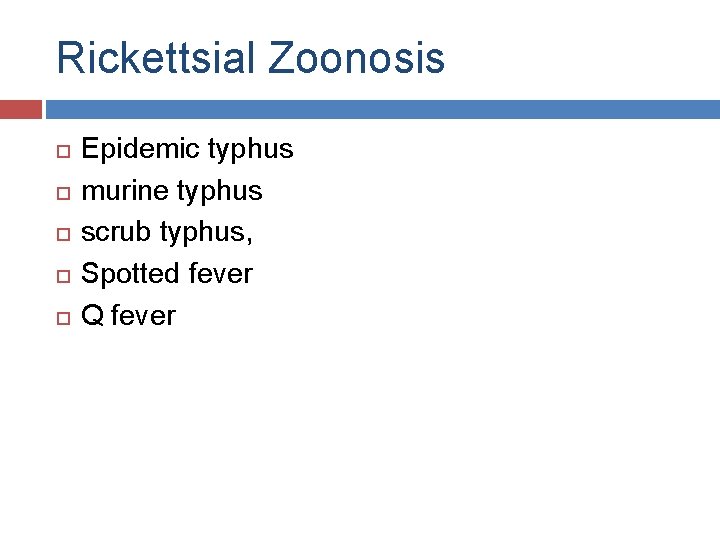
Rickettsial Zoonosis Epidemic typhus murine typhus scrub typhus, Spotted fever Q fever
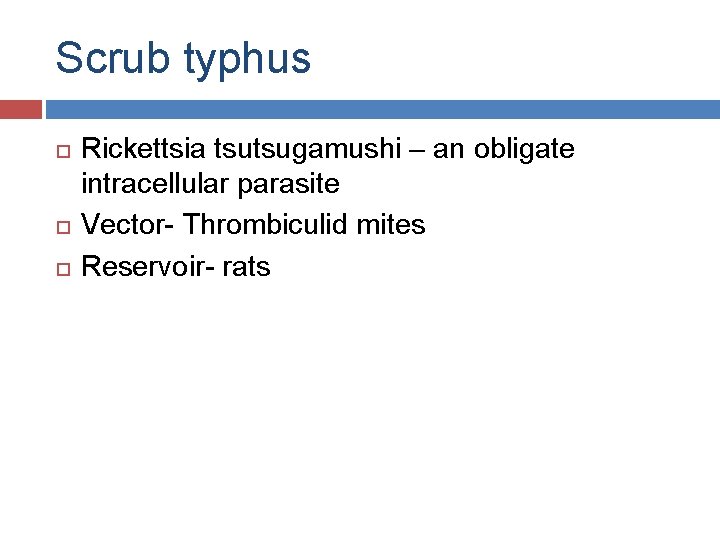
Scrub typhus Rickettsia tsutsugamushi – an obligate intracellular parasite Vector- Thrombiculid mites Reservoir- rats
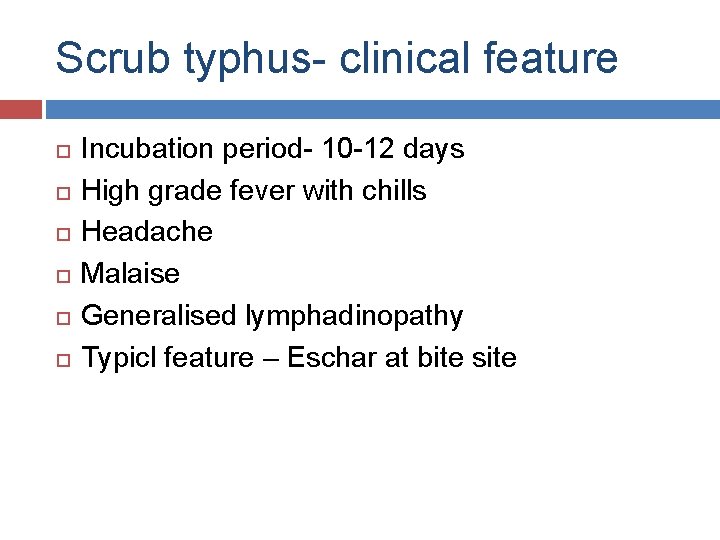
Scrub typhus- clinical feature Incubation period- 10 -12 days High grade fever with chills Headache Malaise Generalised lymphadinopathy Typicl feature – Eschar at bite site
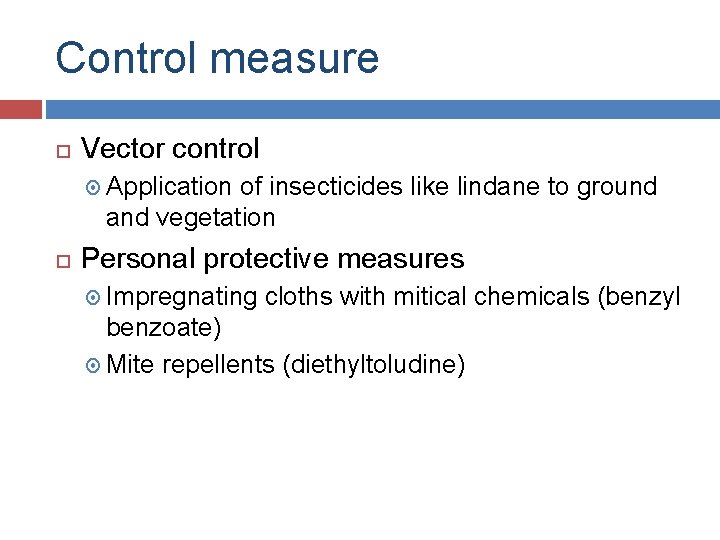
Control measure Vector control Application of insecticides like lindane to ground and vegetation Personal protective measures Impregnating cloths with mitical chemicals (benzyl benzoate) Mite repellents (diethyltoludine)
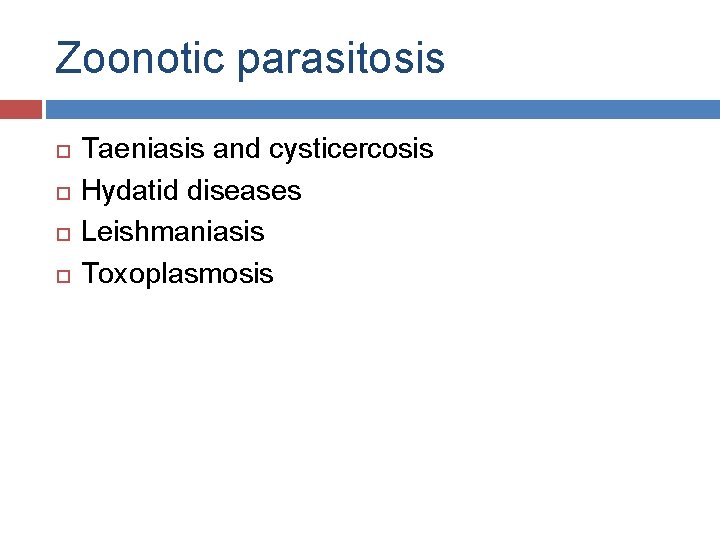
Zoonotic parasitosis Taeniasis and cysticercosis Hydatid diseases Leishmaniasis Toxoplasmosis
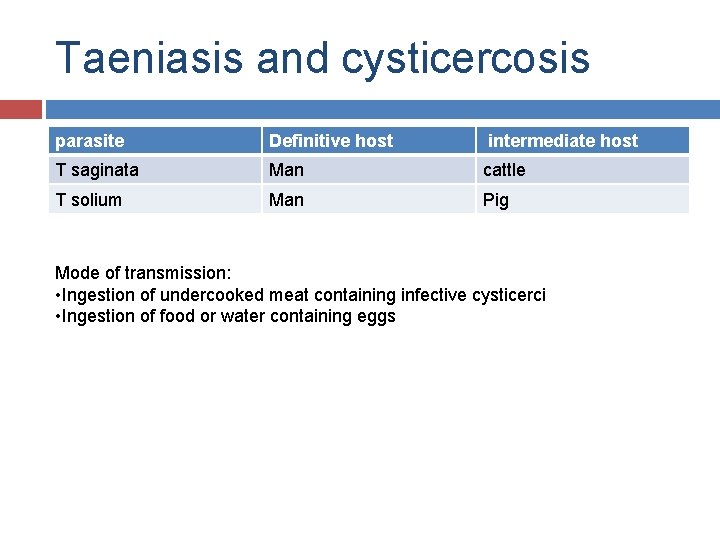
Taeniasis and cysticercosis parasite Definitive host intermediate host T saginata Man cattle T solium Man Pig Mode of transmission: • Ingestion of undercooked meat containing infective cysticerci • Ingestion of food or water containing eggs

Control measures Treatment of infected person using (albendazol/praziquantel) Meat inspection Adequate sewage disposal Thorough cooking of beef and pork

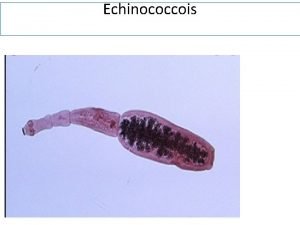
Hydatid Metacystode stage of dog inestinal tape worm (Echinococcus) Prevalent in Tamil nadu and Andra pradesh Dog –sheep/cattle/goat cycle (accidental in man) Human infection Ingestion of eggs through food _ unwashed vegetables Unwashed hand
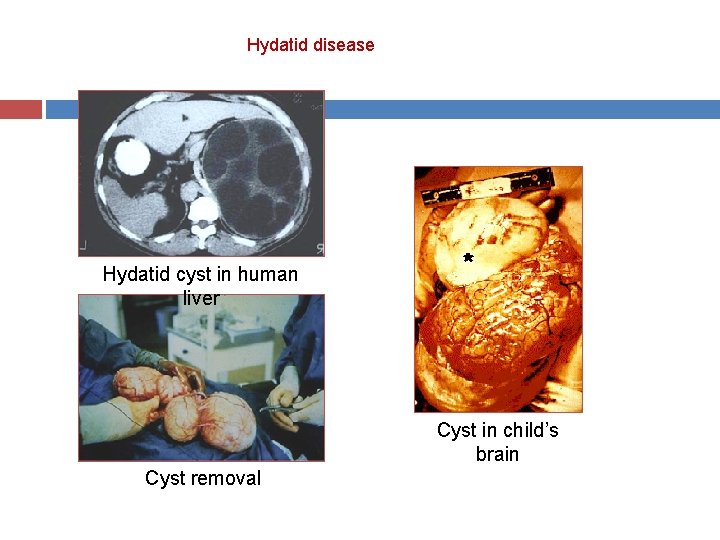
Hydatid disease Hydatid cyst in human liver Cyst in child’s brain Cyst removal

Control Preventing dogs from gaining access to slaughter house waste Control of dog Single dose of prazequental 5 mg/kg for dogs Health education for do owners

Leishmaniasis Organism- Leishmania Vector- Female Phlebotomine sand fly Types Kala azar – L. donovani Cutaneous leishmaniasis-= L. Tropica Muco cutaneous- L. brazilensis Reservoir- dogs, jackals, fox, rodents In india Kal Azal in mainly non zootic

ZOONOSES PREVENTION Sensible personal hygiene • Wash hands after handling animals • Wash hands before eating or drinking • Disinfect and dress wounds as soon as possible • Wash eyes if urine splashed Necropsy • Wear gloves and a face mask if unsure Working with sick wildlife • Take special precautions e. g. Personal protective equipment (PPE) Immunization and chemoprophylaxis when required Slide 73

Thank you
- Slides: 72