You love Chemistry Chapter 17 Additional Aspects of










![Solubility Products The equilibrium constant expression for this equilibrium is Ksp = [Ba 2+] Solubility Products The equilibrium constant expression for this equilibrium is Ksp = [Ba 2+]](https://slidetodoc.com/presentation_image_h/7d068cd4318837aa1295a2112cecd0f8/image-17.jpg)















- Slides: 37

You love Chemistry Chapter 17 Additional Aspects of Aqueous Equilibria You might have doubts, but deep, deep down you know there is a little love for the central science! Aqueous Equilibria

How do they effect dissociation? THE COMMON ION EFFECT Aqueous Equilibria

Compare: • Calculate the p. H of a 0. 25 M propionic acid solution that solution (Ka=1. 3 x 10 -5) also has 0. 10 M sodium propionate add. Aqueous Equilibria

Common Ion Effect • How does Le. Chatelier support the previous calculations? Aqueous Equilibria

Common Ion Effect • Summarize the Common Ion Effect: Aqueous Equilibria

Buffers: • Solutions of a weak conjugate acid-base pair. • They are particularly resistant to p. H changes, even when strong acid or base is added. Aqueous Equilibria

How do Buffers Resist p. H Changes? • Consider a buffer composed of equal concentrations of nitrous acid and nitrite ion. • 1) Does this meet the criteria for a buffer? • 2) What would happen if a volume of HCl was added to the buffer? • 3) What would happen if a volume of Na. OH was added to the buffer Aqueous Equilibria

How do Buffers Resist p. H Changes? • The p. H of a buffer will change somewhat, but not significantly. Ø The Balance between the conjugate acid/base pair is disrupted Ø Either the conjugate acid or the conjugate base will be present in a higher concentration after the addition Ø This will cause a minor change to the p. H Aqueous Equilibria

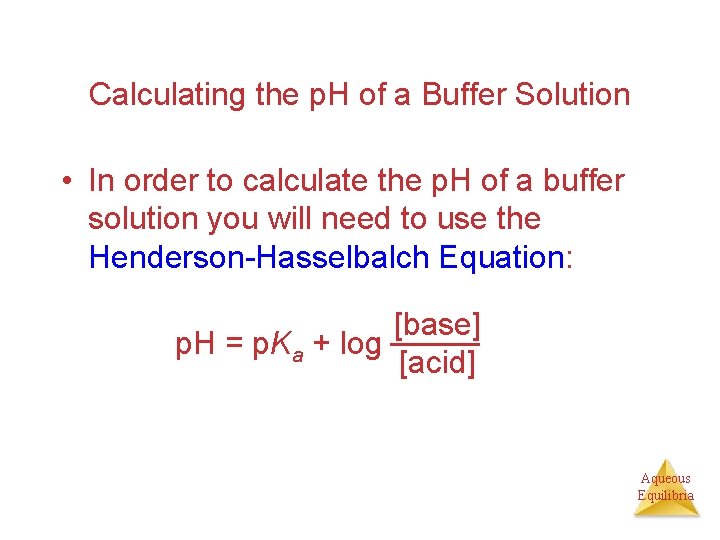
Calculating the p. H of a Buffer Solution • In order to calculate the p. H of a buffer solution you will need to use the Henderson-Hasselbalch Equation: [base] p. H = p. Ka + log [acid] Aqueous Equilibria

Henderson–Hasselbalch Equation What is the p. H of a buffer that is 0. 12 M in benzoic acid and 0. 20 M in sodium benzoate? Ka for benzoic acid is 6. 3 10− 5. Aqueous Equilibria

Another Application of the Henderson -Hasselbalch Equation • How many moles of NH 4 Cl must be added to 2. 0 L of 0. 10 M NH 3 to form a buffer whose p. H is 9. 00? (Assume that the addition of NH 4 Cl does not change the volume of the solution. ) Kb for NH 3 is 1. 8 x 10 -5. Aqueous Equilibria

p. H Range and Buffer Capacity • The p. H range is the range of p. H values over which a buffer system works effectively. • It is best to choose an acid with a p. Ka close to the desired p. H. • Buffer Capacity is the amount of acid or base a buffer can neutralize before significant p. H changes Ø The higher the molarity or volume of the conjugate pairs, the greater the capacity of the buffer. Aqueous Equilibria

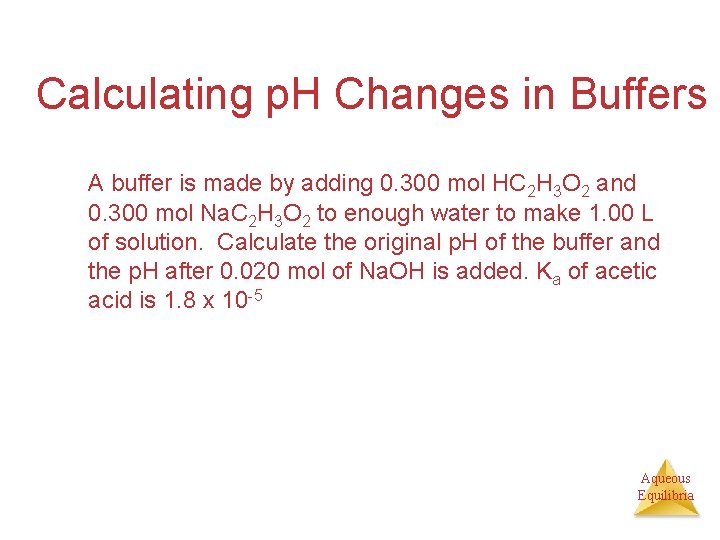
Calculating p. H Changes in Buffers A buffer is made by adding 0. 300 mol HC 2 H 3 O 2 and 0. 300 mol Na. C 2 H 3 O 2 to enough water to make 1. 00 L of solution. Calculate the original p. H of the buffer and the p. H after 0. 020 mol of Na. OH is added. Ka of acetic acid is 1. 8 x 10 -5 Aqueous Equilibria

Homework • Ch. 17: 15, 17, 21, 23, 25 Aqueous Equilibria

Solubility Product Constant Using Equilibrium to Determine the dissociation of a solid in solution. Aqueous Equilibria

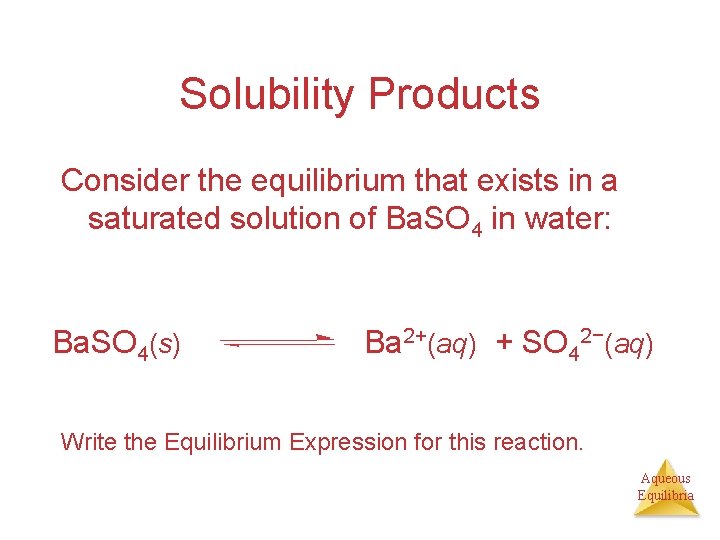
Solubility Products Consider the equilibrium that exists in a saturated solution of Ba. SO 4 in water: Ba. SO 4(s) Ba 2+(aq) + SO 42−(aq) Write the Equilibrium Expression for this reaction. Aqueous Equilibria
![Solubility Products The equilibrium constant expression for this equilibrium is Ksp Ba 2 Solubility Products The equilibrium constant expression for this equilibrium is Ksp = [Ba 2+]](https://slidetodoc.com/presentation_image_h/7d068cd4318837aa1295a2112cecd0f8/image-17.jpg)
Solubility Products The equilibrium constant expression for this equilibrium is Ksp = [Ba 2+] [SO 42−] where the equilibrium constant, Ksp, is called the solubility product. The solubility product defines the dissociation of the solid in solution Aqueous Equilibria

Practice Problem • Write separate expressions for the solubility product constant for Ca. F 2 and Silver Sulfate Aqueous Equilibria

Practice Problem • Determine the concentration of each ion in a saturated solution of zinc hydroxide. Zinc hydroxide has a Ksp=3. 0 x 10 -16 Aqueous Equilibria

Solubility Products • Ksp is not the same as solubility. • Solubility is generally expressed as the mass of solute dissolved in 1 L (g/L) or 100 m. L (g/m. L) of solution, or in mol/L (M). Aqueous Equilibria

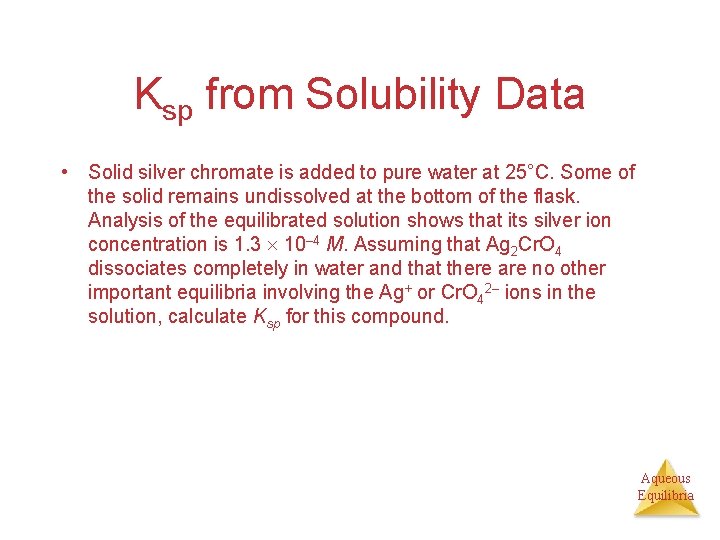
Ksp from Solubility Data • Solid silver chromate is added to pure water at 25°C. Some of the solid remains undissolved at the bottom of the flask. Analysis of the equilibrated solution shows that its silver ion concentration is 1. 3 10– 4 M. Assuming that Ag 2 Cr. O 4 dissociates completely in water and that there are no other important equilibria involving the Ag+ or Cr. O 42– ions in the solution, calculate Ksp for this compound. Aqueous Equilibria

Solubility from Ksp • The Ksp for Ca. F 2 is 3. 9 10– 11 at 25°C. Assuming that Ca. F 2 dissociates completely upon dissolving and that there are no other important equilibria affecting its solubility, calculate the solubility of Ca. F 2 in grams per liter. Aqueous Equilibria

Refresher Problems Getting our mind back into Chemistry Mode. Aqueous Equilibria

Equilibrium Expressions • Write equilibrium expressions for the following reactions: • 1) Ni(OH)2(s) D Ni 2+(aq) + 2 OH-(aq) • 2) 2 NOBr(g) D 2 NO(g) + Br 2(g) • 3) HCl. O 3(ag) H+(aq) + Cl. O 3 -(aq) • 4) NH 3(aq) + H 2 O(l) DNH 4+(aq) + OH-(aq) Aqueous Equilibria

p. H • What is the p. H of a solution that consists of 250 ml of 0. 75 M hydrofluoric acid and 2. 1 grams of sodium fluoride? (Ka=6. 8 x 10 -4) Aqueous Equilibria

Solubility • What’s the molar concentration of each ion in an equilibrated solution of lead (II) fluoride. Ksp of lead (II) fluoride= 3. 6 x 10 -8 • Can we figure out the p. H of this solution? Aqueous Equilibria

Buffer Solutions • What is the p. H of a 500. 0 m. L buffer solution that consists of 1. 25 M acetic acid and 1. 00 M sodium acetate if 15. 0 m. L of 0. 750 M nitric acid is added to it? Ka of acetic acid is 1. 80 x 10 -5 Aqueous Equilibria

Read and Take Notes on section 17. 3 Acid. Base Titrations HOMEWORK Aqueous Equilibria

Factors that Affect Solubility Aqueous Equilibria

Factors that Affect Solubility • From your understanding of equilibrium and how it relates to acids/bases, what would be some factors that either increase or decrease the dissociation of a solid in solution? Aqueous Equilibria

Factors Affecting Solubility 1) The Common-Ion Effect Ø If one of the ions in a solution equilibrium is already dissolved in the solution, the equilibrium will shift to the left and the solubility of the salt will decrease. Ba. SO 4(s) Ba 2+(aq) + SO 42−(aq) Aqueous Equilibria

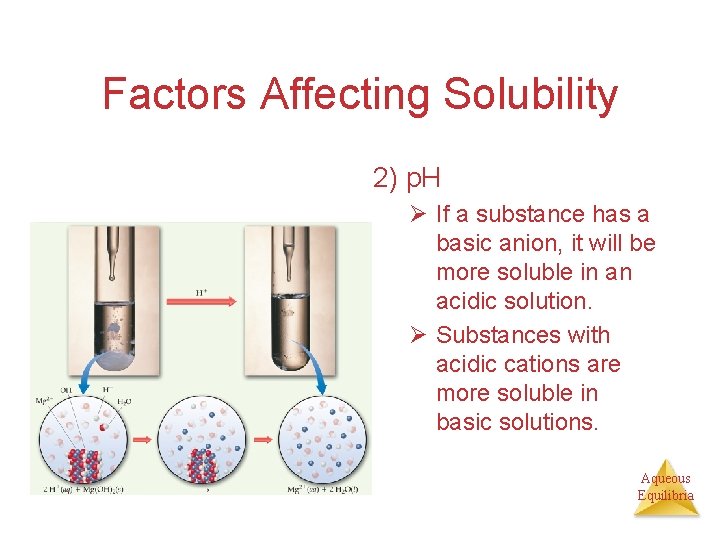
Factors Affecting Solubility 2) p. H Ø If a substance has a basic anion, it will be more soluble in an acidic solution. Ø Substances with acidic cations are more soluble in basic solutions. Aqueous Equilibria

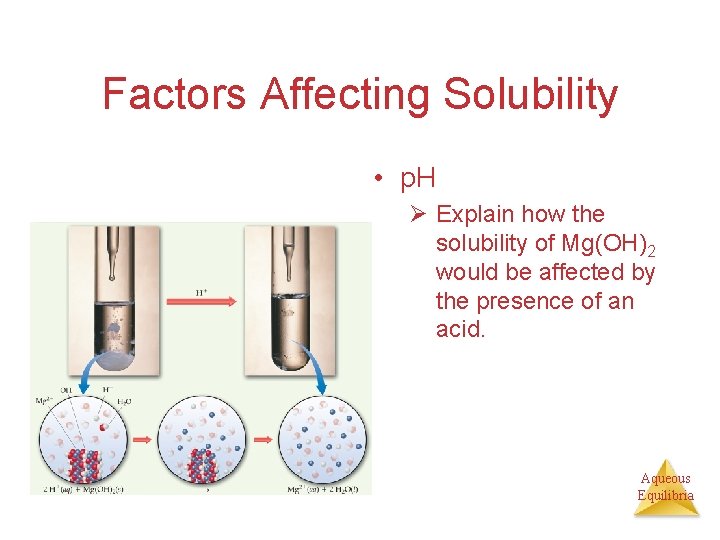
Factors Affecting Solubility • p. H Ø Explain how the solubility of Mg(OH)2 would be affected by the presence of an acid. Aqueous Equilibria

Practice Problem • Which of the following substances will be more soluble in acidic solution than in basic solution: (a) Ni(OH)2(s) (b) Ca. CO 3(s) (c) Ag. Cl(s) (d) Ba. F 2(s) Aqueous Equilibria

Evaluating a Solution Will more solid dissolve or will a precipitate form? Aqueous Equilibria

Will a Precipitate Form? • In a solution, Ø If Q = Ksp, the system is at equilibrium and the solution is saturated. Ø If Q < Ksp, more solid will dissolve until Q = Ksp. Ø If Q > Ksp, the salt will precipitate until Q = Ksp. Aqueous Equilibria

Practice Problem • Will a precipitate form when 0. 10 L of 8. 0 10 – 3 M Pb(NO ) is added to 0. 40 L of 5. 0 10– 3 3 2 M Na 2 SO 4? (Ksp for Pb. SO 4=6. 3 x 10 -9) Aqueous Equilibria