Yesterdays Homework Questions p 148 1 5 p







































- Slides: 59

Yesterday’s Homework Questions: p. 148 #1 -5 p. 150 #1 -3 p. 153 #10, 11

Ions! SNC 2 D

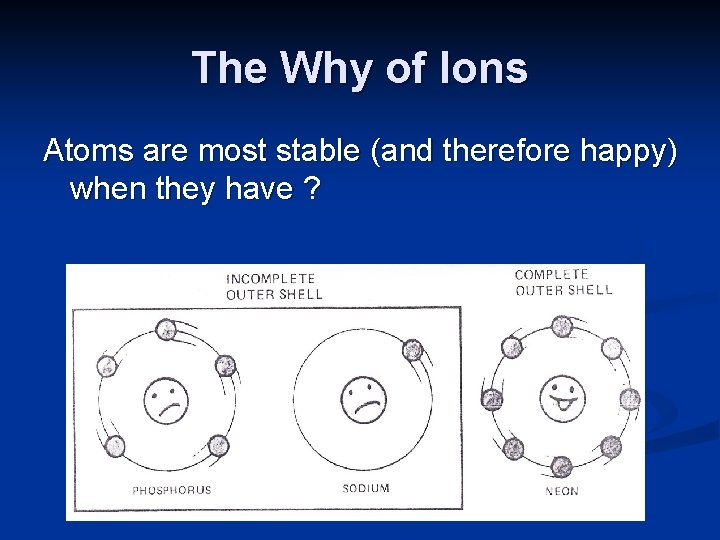
The Why of Ions Atoms are most stable (and therefore happy) when they have ?

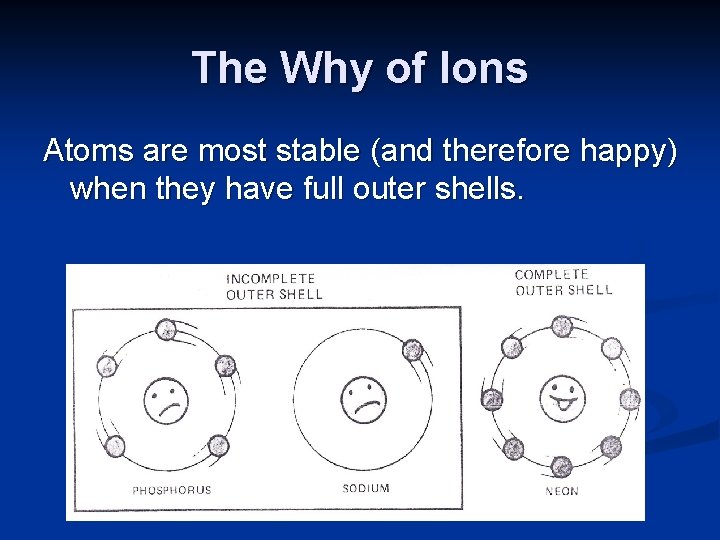
The Why of Ions Atoms are most stable (and therefore happy) when they have full outer shells.

The How of Ions Atoms that have fewer electrons in their outer shells than it would take to fill that shell will preferentially lose electrons.

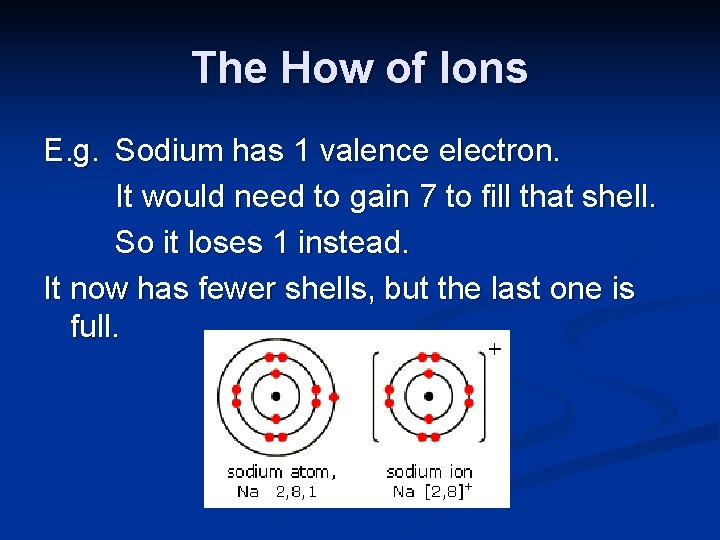
The How of Ions E. g. Sodium has 1 valence electron. It would need to gain 7 to fill that shell. So it loses 1 instead. It now has fewer shells, but the last one is full.

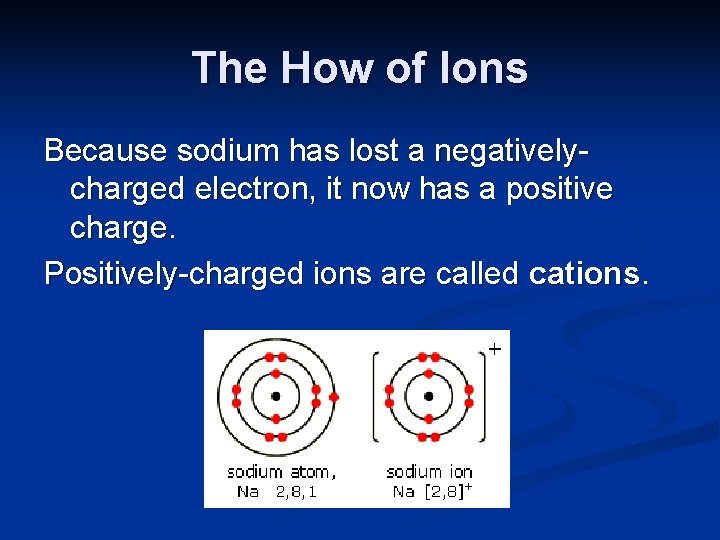
The How of Ions Because sodium has lost a negativelycharged electron, it now has a ?

The How of Ions Because sodium has lost a negativelycharged electron, it now has a positive charge. Positively-charged ions are called cations.

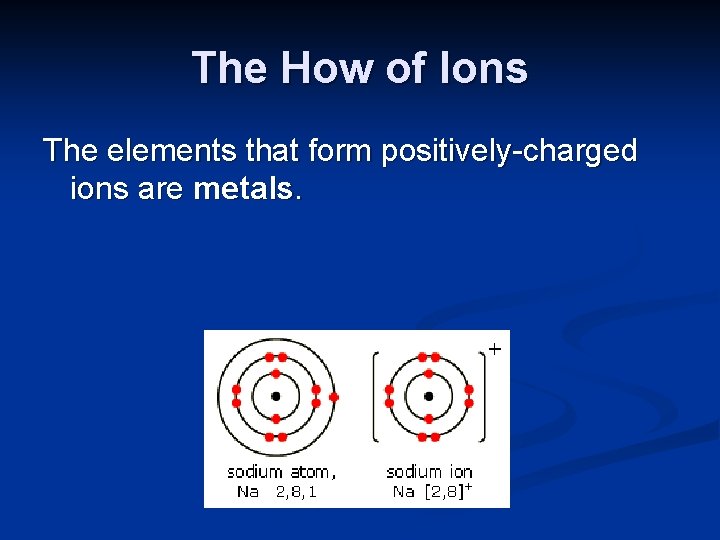
The How of Ions The elements that form positively-charged ions are metals.

Valence Charge The charge on an ion is said to be its valence charge, or simply valence. E. g. The valence of sodium is ?

Valence Charge The charge on an ion is said to be its valence charge, or simply valence. E. g. The valence of sodium is +1 or 1+. Let’s look at some more metal ions. .

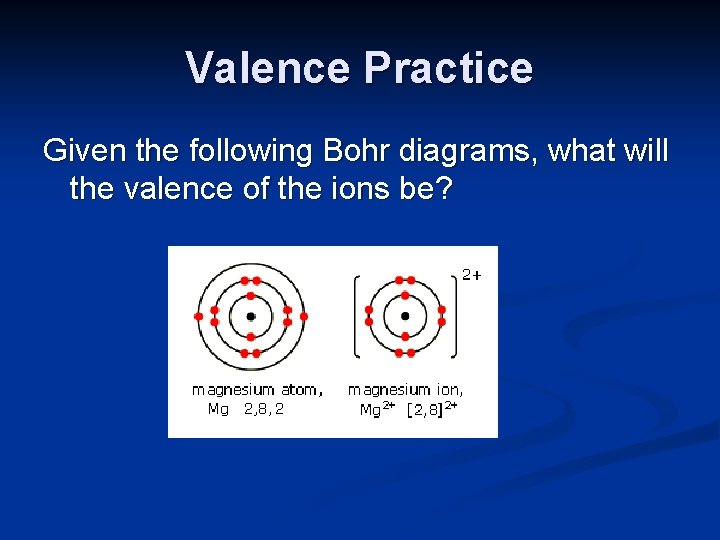
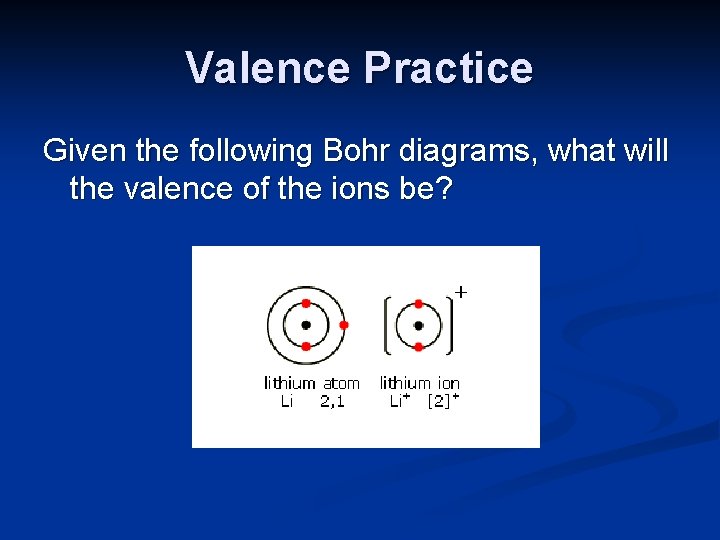
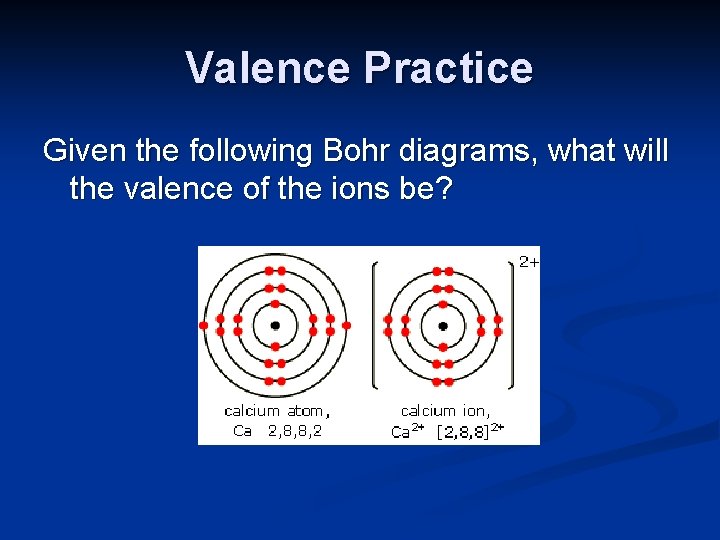
Valence Practice Given the following Bohr diagrams, what will the valence of the ions be?

Valence Practice Given the following Bohr diagrams, what will the valence of the ions be?

Valence Practice Given the following Bohr diagrams, what will the valence of the ions be?

Valence Practice Given the following Bohr diagrams, what will the valence of the ions be?

Valence Practice Given the following Bohr diagrams, what will the valence of the ions be?

Valence Practice Given the following Bohr diagrams, what will the valence of the ions be?

Valence Charge Note that sodium and lithium, both in the 1 st column, have a valence of 1+. And magnesium and calcium, both in the 2 nd column, have a valence of 2+. Since elements in the same column or family on the periodic table have the same number of ?

Valence Charge Note that sodium and lithium, both in the 1 st column, have a valence of 1+. And magnesium and calcium, both in the 2 nd column, have a valence of 2+. Since elements in the same column or family on the periodic table have the same number of valence electrons, they will typically form ions in the same way and have the same valence charge.

Valence Charge Some metals can form ions in two different ways and have two possible valences; these metals are said to be ?

Valence Charge Some metals can form ions in two different ways and have two possible valences; these metals are said to be multivalent. E. g. the valence of lead is ?

Valence Charge Some metals can form ions in two different ways and have two possible valences; these metals are said to be multivalent. E. g. the valence of lead is 2+ or 4+. To indicate which ion we are dealing with, we write the valence charge in Roman numerals after the name of the metal. E. g. lead (? ) or lead (? )

Valence Charge Some metals can form ions in two different ways and have two possible valences; these metals are said to be multivalent. E. g. the valence of lead is 2+ or 4+. To indicate which ion we are dealing with, we write the valence charge in Roman numerals after the name of the metal. E. g. lead (II) or lead (? )

Valence Charge Some metals can form ions in two different ways and have two possible valences; these metals are said to be multivalent. E. g. the valence of lead is 2+ or 4+. To indicate which ion we are dealing with, we write the valence charge in Roman numerals after the name of the metal. E. g. lead (II) or lead (IV)

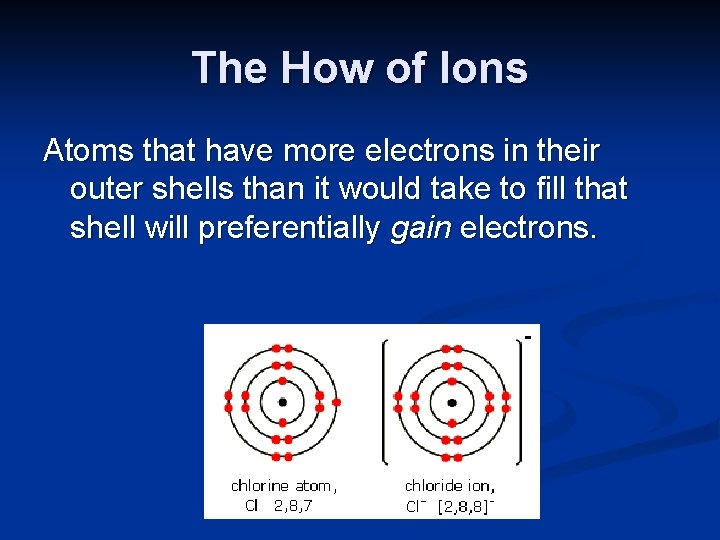
The How of Ions Atoms that have more electrons in their outer shells than it would take to fill that shell will preferentially gain electrons.

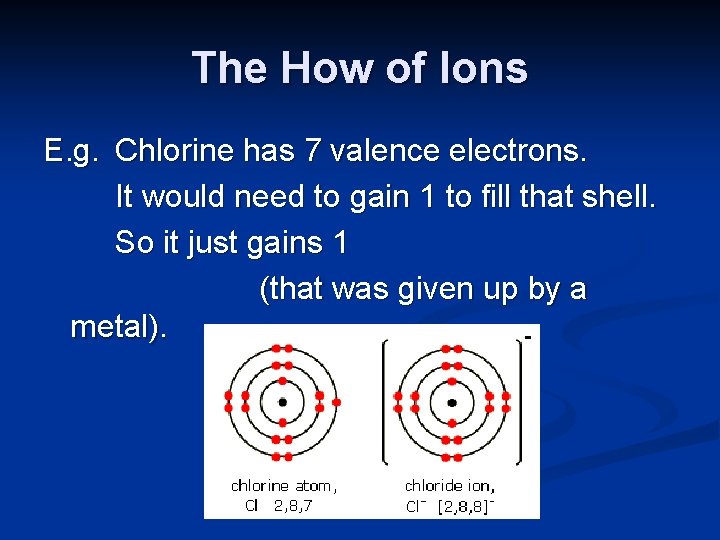
The How of Ions E. g. Chlorine has 7 valence electrons. It would need to gain 1 to fill that shell. So it just gains 1 (that was given up by a metal).

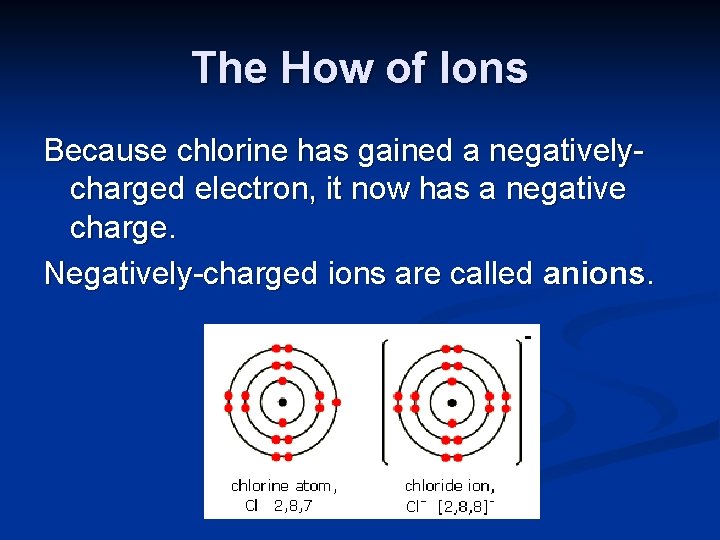
The How of Ions Because chlorine has gained a negativelycharged electron, it now has a negative charge. Negatively-charged ions are called anions.

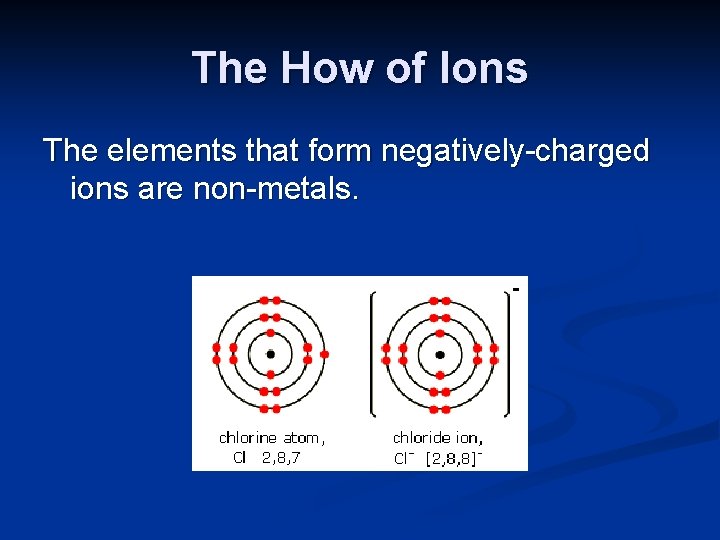
The How of Ions The elements that form negatively-charged ions are non-metals.

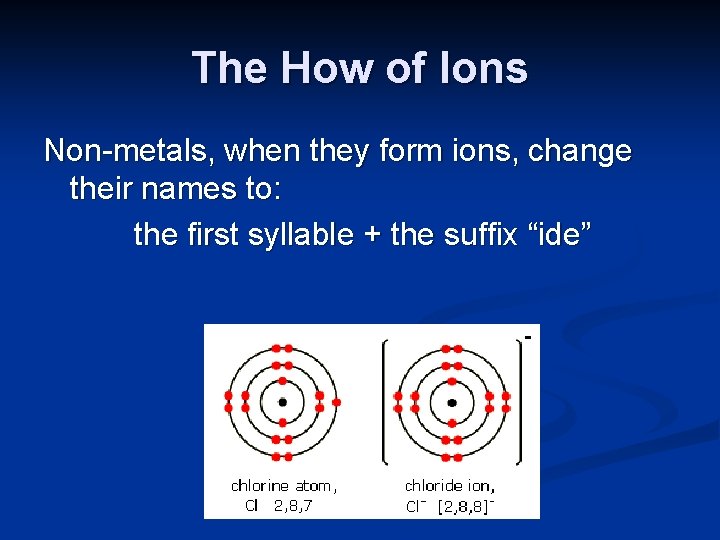
The How of Ions Non-metals, when they form ions, change their names to: the first syllable + the suffix “ide”

Anion names chlorine fluorine bromine oxygen sulphur nitrogen phosphorus

Anion names chlorine fluorine bromine oxygen sulphur nitrogen phosphorus chloride

Anion names chlorine fluorine bromine oxygen sulphur nitrogen phosphorus chloride fluoride

Anion names chlorine fluorine bromine oxygen sulphur nitrogen phosphorus chloride fluoride bromide

Anion names chlorine fluorine bromine oxygen sulphur nitrogen phosphorus chloride fluoride bromide oxide

Anion names chlorine fluorine bromine oxygen sulphur nitrogen phosphorus chloride fluoride bromide oxide sulphide

Anion names chlorine fluorine bromine oxygen sulphur nitrogen phosphorus chloride fluoride bromide oxide sulphide nitride

Anion names chlorine fluorine bromine oxygen sulphur nitrogen phosphorus chloride fluoride bromide oxide sulphide nitride phosphide

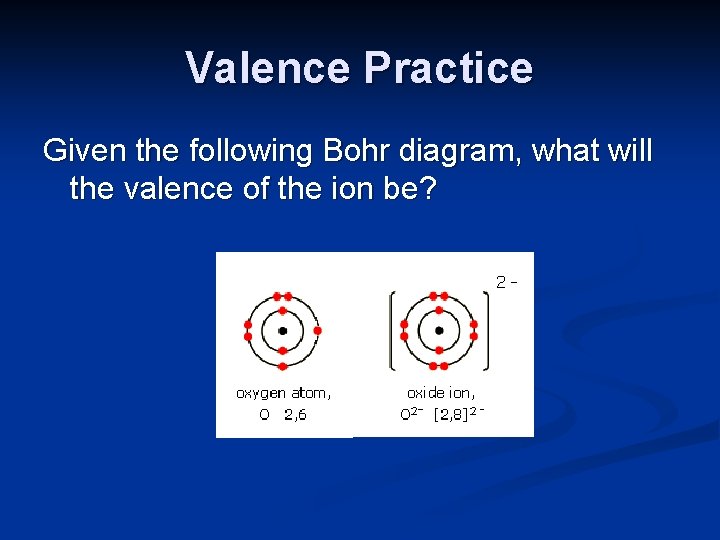
Valence Practice Given the following Bohr diagram, what will the valence of the ion be?

Valence Practice Given the following Bohr diagram, what will the valence of the ion be?

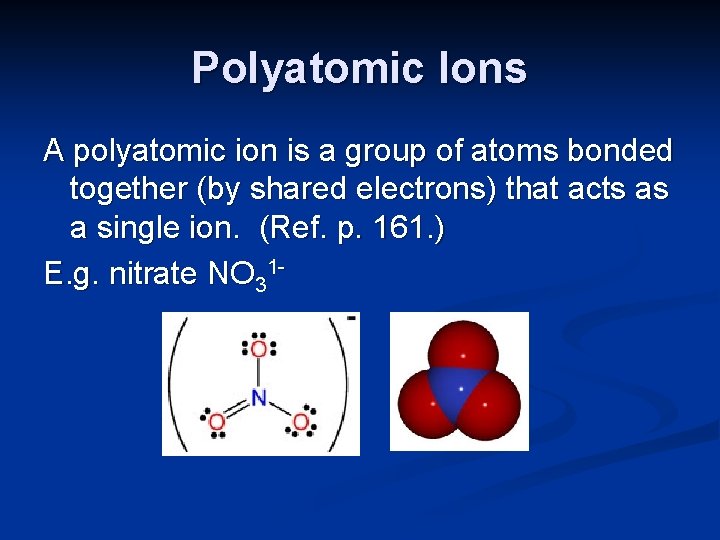
Polyatomic Ions A polyatomic ion is a group of atoms bonded together (by shared electrons) that acts as a single ion. (Ref. p. 161. ) E. g. nitrate NO 31 -

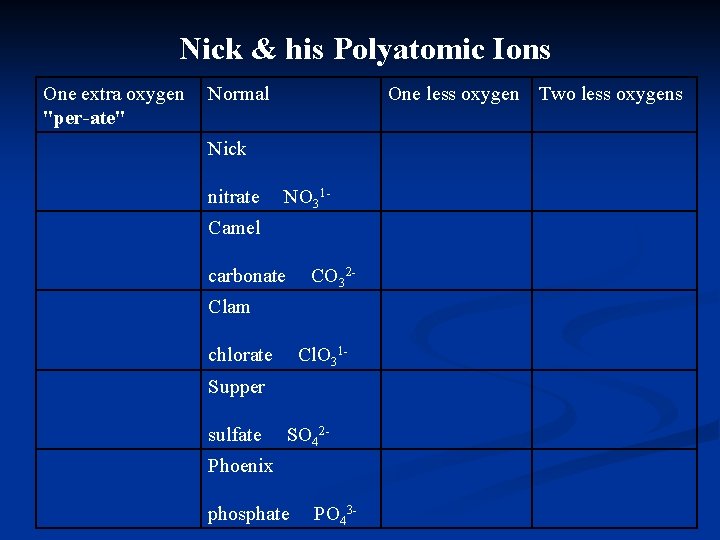
Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix First consonants = Name of the ion e. g. N from Nick stands for:

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix First consonants = Name of the ion e. g. N from Nick stands for: Nitrate

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix First consonants = Name of the ion # of vowels = valence e. g. Nick has 1 vowel so it has:

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix First consonants = Name of the ion # of vowels = valence e. g. Nick has 1 vowel so it has: a charge of 1 -

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix First consonants = Name of the ion # of vowels = valence # of consonants = # of oxygens e. g. Nick has 3 consonants so it has:

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix First consonants = Name of the ion # of vowels = valence # of consonants = # of oxygens e. g. Nick has 3 consonants so it has: 3 oxygens

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix Write the formulae for the corresponding ions Nick = Camel = Clam = Supper = Phoenix =

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix Write the formulae for the corresponding ions Nick = nitrate NO 31 Camel = Clam = Supper = Phoenix =

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix Write the formulae for the corresponding ions Nick = nitrate NO 31 Camel = carbonate CO 32 Clam = Supper = Phoenix =

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix Write the formulae for the corresponding ions Nick = nitrate NO 31 Camel = carbonate CO 32 Clam = chlorate Cl. O 31 Supper = Phoenix =

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix Write the formulae for the corresponding ions Nick = nitrate NO 31 Camel = carbonate CO 32 Clam = chlorate Cl. O 31 Supper = sulfate SO 42 Phoenix =

Nick & his Polyatomic Ions Nick the Camel had a Clam for Supper in Phoenix Write the formulae for the corresponding ions Nick = nitrate NO 31 Camel = carbonate CO 32 Clam = chlorate Cl. O 31 Supper = sulfate SO 42 Phoenix = phosphate PO 43 -

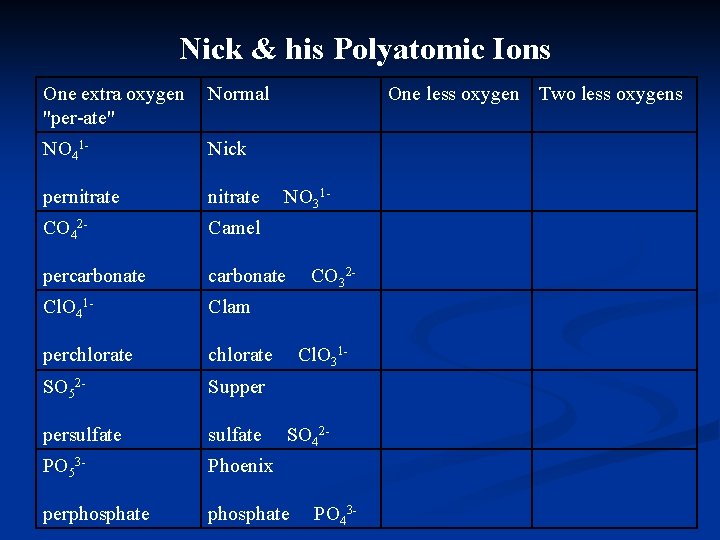
Nick & his Polyatomic Ions One extra oxygen "per-ate" Normal One less oxygen Two less oxygens Nick nitrate NO 31 - Camel carbonate CO 32 - Clam chlorate Cl. O 31 - Supper sulfate SO 42 - Phoenix phosphate PO 43 -

Nick & his Polyatomic Ions One extra oxygen "per-ate" Normal One less oxygen Two less oxygens NO 41 - Nick pernitrate CO 42 - Camel percarbonate Cl. O 41 - Clam perchlorate SO 52 - Supper persulfate PO 53 - Phoenix perphosphate NO 31 - CO 32 - Cl. O 31 - SO 42 - PO 43 -

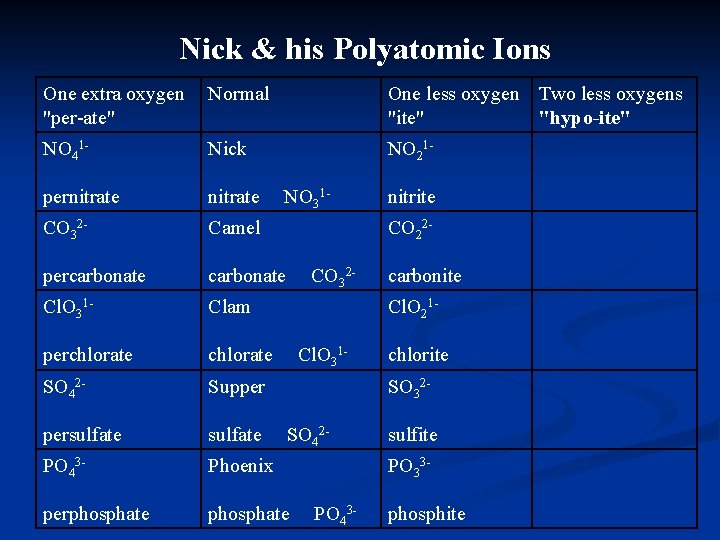
Nick & his Polyatomic Ions One extra oxygen "per-ate" Normal One less oxygen Two less oxygens "ite" "hypo-ite" NO 41 - Nick NO 21 - pernitrate CO 32 - Camel percarbonate Cl. O 31 - Clam perchlorate SO 42 - Supper persulfate PO 43 - Phoenix perphosphate NO 31 - nitrite CO 22 - CO 32 - carbonite Cl. O 21 - Cl. O 31 - chlorite SO 32 - SO 42 - sulfite PO 33 - PO 43 - phosphite

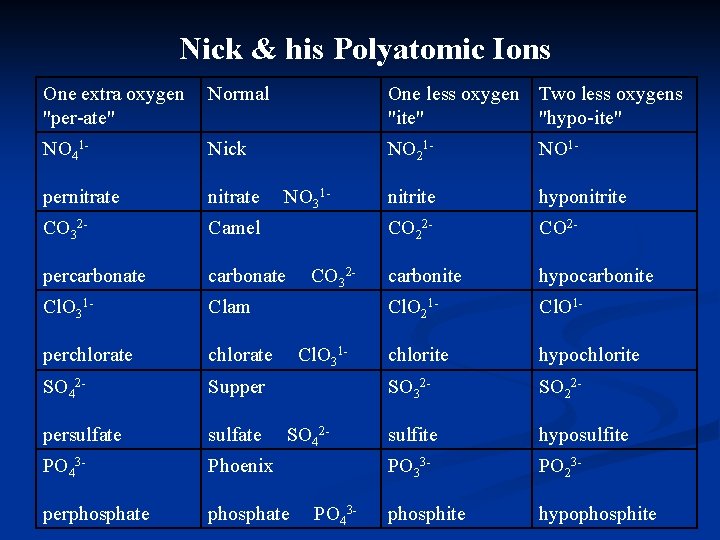
Nick & his Polyatomic Ions One extra oxygen "per-ate" Normal One less oxygen Two less oxygens "ite" "hypo-ite" NO 41 - Nick NO 21 - NO 1 - pernitrate nitrite hyponitrite CO 32 - Camel CO 22 - CO 2 - percarbonate carbonite hypocarbonite Cl. O 31 - Clam Cl. O 21 - Cl. O 1 - perchlorate chlorite hypochlorite SO 42 - Supper SO 32 - SO 22 - persulfate sulfite hyposulfite PO 43 - Phoenix PO 33 - PO 23 - perphosphate phosphite hypophosphite NO 31 - CO 32 - Cl. O 31 - SO 42 - PO 43 -

Three More You should also be familiar with: HCO 3 hydrogen carbonate (or bicarbonate) OH- hydroxide NH 4+ ammonium

Tune in next time Tomorrow we will discuss ionic compounds and solutions of ionic compounds – and we will test for ions in solutions. For now, practice getting to know your ions with ion flash cards.