Yeast Colony PCR provides a forensics tool for
- Slides: 16

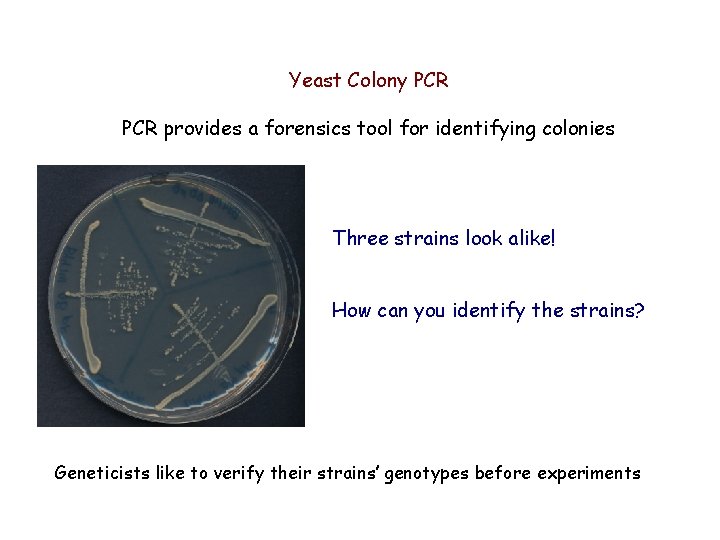
Yeast Colony PCR provides a forensics tool for identifying colonies Three strains look alike! How can you identify the strains? Geneticists like to verify their strains’ genotypes before experiments

Discovery of the polymerase chain reaction expanded the reach of molecular biologists Key discovery: DNA polymerases from Thermus aquaticus and othermophiles are active at high temperatures and do not denature at temperatures that denature DNA helices Photo by Yellowstone NPS PCR can generate a billion copies of a DNA sequence of interest Investigators can generate custom DNA sequences with PCR is also a discovery tool, since only partial sequence information is needed to design primers

What principles are used to design PCR primers? What happens at each temperature in a PCR cycle? How can PCR be used to identify MET genes that have been disrupted by the insertion of a KANR cassette?

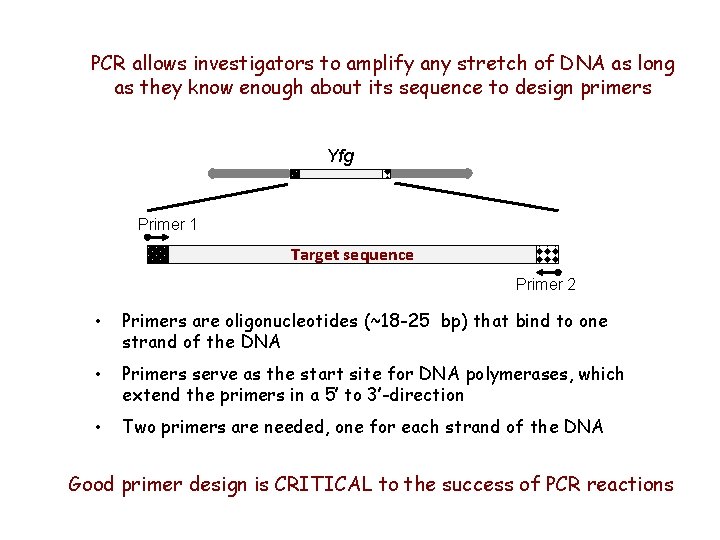
PCR allows investigators to amplify any stretch of DNA as long as they know enough about its sequence to design primers Yfg Primer 1 Target sequence Primer 2 • Primers are oligonucleotides (~18 -25 bp) that bind to one strand of the DNA • Primers serve as the start site for DNA polymerases, which extend the primers in a 5’ to 3’-direction • Two primers are needed, one for each strand of the DNA Good primer design is CRITICAL to the success of PCR reactions

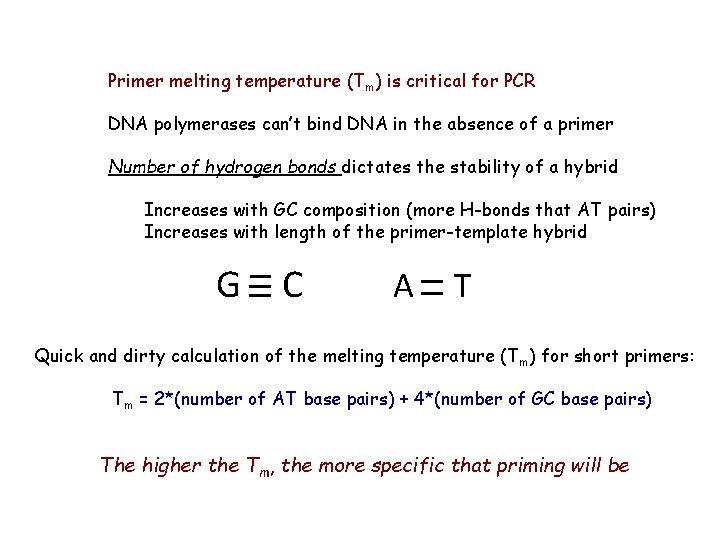
Primer melting temperature (Tm) is critical for PCR DNA polymerases can’t bind DNA in the absence of a primer Number of hydrogen bonds dictates the stability of a hybrid Increases with GC composition (more H-bonds that AT pairs) Increases with length of the primer-template hybrid G C A T Quick and dirty calculation of the melting temperature (T m) for short primers: Tm = 2*(number of AT base pairs) + 4*(number of GC base pairs) The higher the Tm, the more specific that priming will be

What principles are used to design PCR primers? What happens at each temperature in a PCR cycle? How can PCR be used to identify MET genes that have been disrupted by the insertion of a KANR cassette?

Researchers use a thermocycler for PCR reactions Programmed to bring the reaction blocks through a series of temperature changes Temperatures typically cycle between: 94 -95˚C - DNA denatures (single-stranded) 55˚C – Primers anneal with target DNA 72 ˚C – Polymerases extend primers, copying DNA

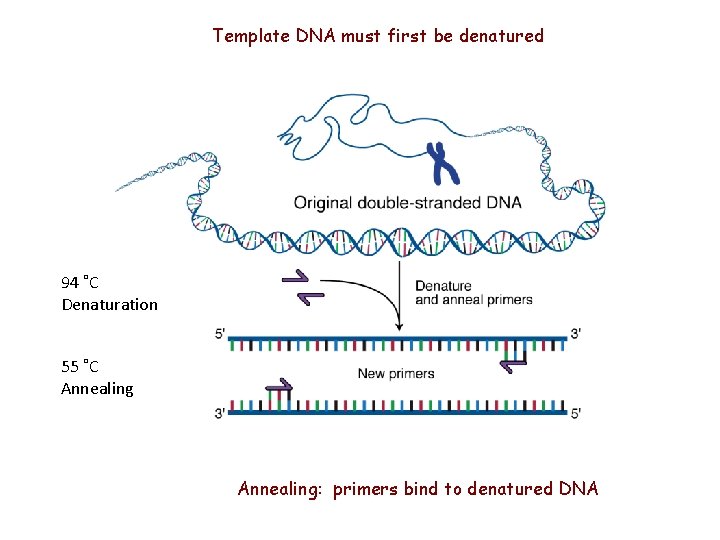
Template DNA must first be denatured 94 ˚C Denaturation 55 ˚C Annealing: primers bind to denatured DNA

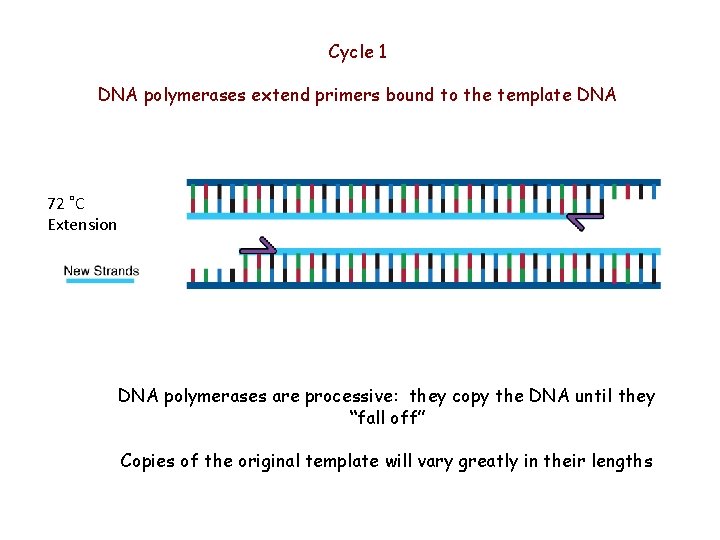
Cycle 1 DNA polymerases extend primers bound to the template DNA 72 ˚C Extension DNA polymerases are processive: they copy the DNA until they “fall off” Copies of the original template will vary greatly in their lengths

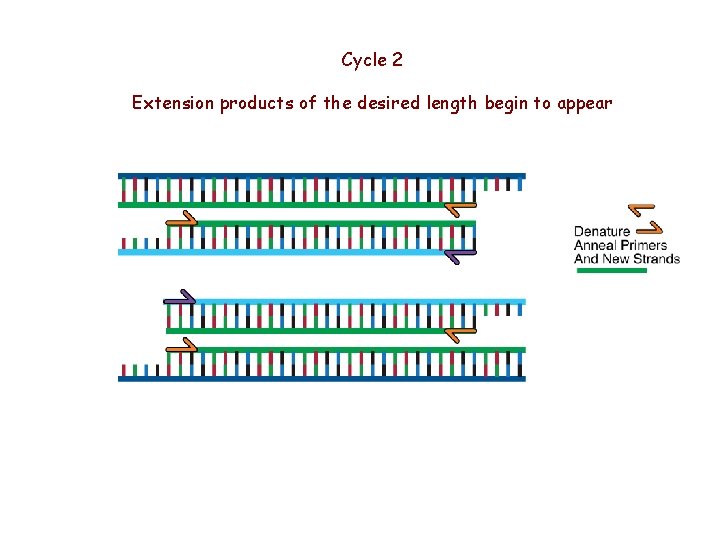
Cycle 2 Extension products of the desired length begin to appear

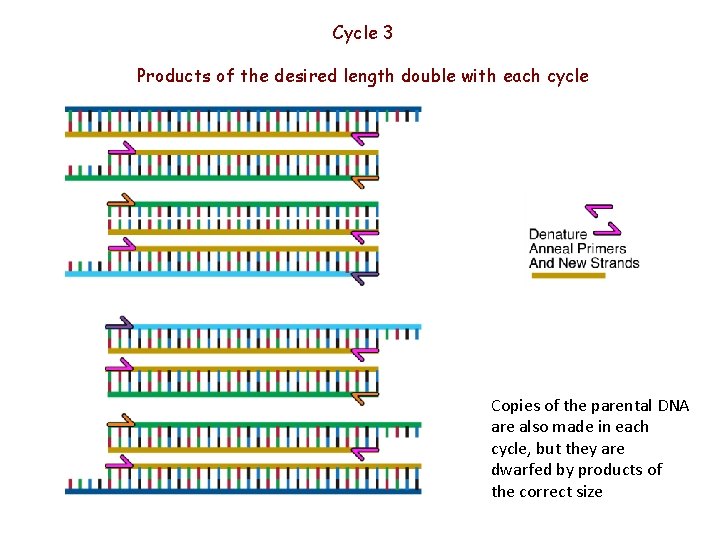
Cycle 3 Products of the desired length double with each cycle Copies of the parental DNA are also made in each cycle, but they are dwarfed by products of the correct size

What principles are used to design PCR primers? What happens at each temperature in a PCR cycle? How can PCR be used to identify MET genes that have been disrupted by the insertion of a KANR cassette?

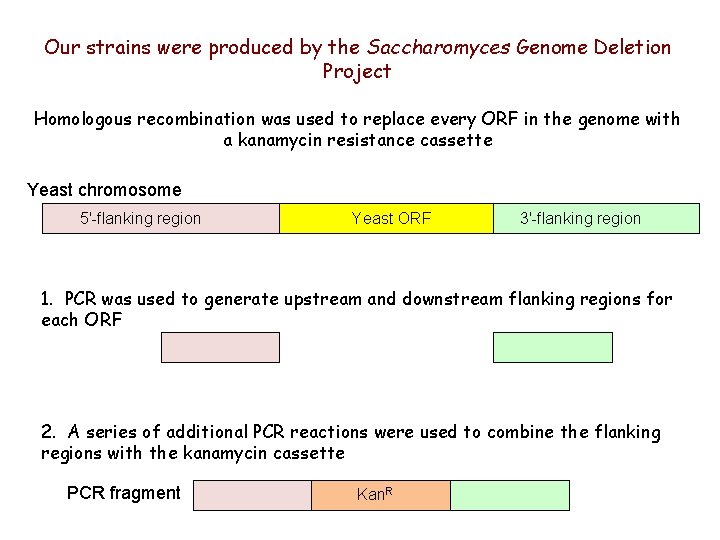
Our strains were produced by the Saccharomyces Genome Deletion Project Homologous recombination was used to replace every ORF in the genome with a kanamycin resistance cassette Yeast chromosome 5'-flanking region Yeast ORF 3'-flanking region 1. PCR was used to generate upstream and downstream flanking regions for each ORF 2. A series of additional PCR reactions were used to combine the flanking regions with the kanamycin cassette PCR fragment Kan. R

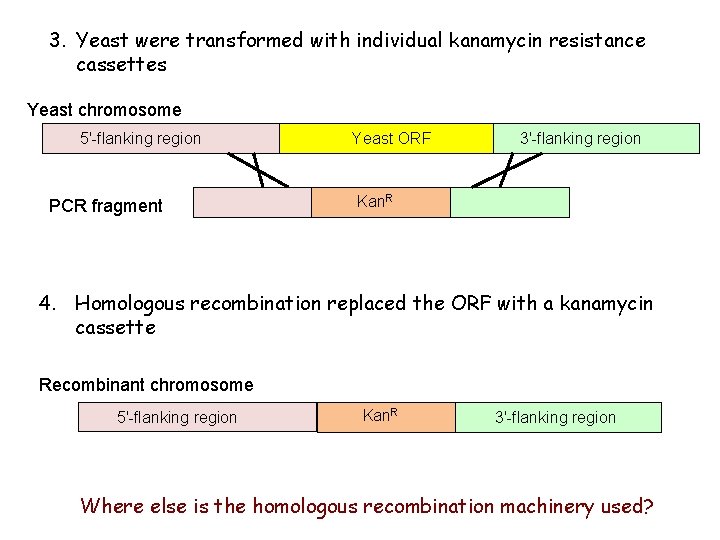
3. Yeast were transformed with individual kanamycin resistance cassettes Yeast chromosome 5'-flanking region PCR fragment Yeast ORF 3'-flanking region Kan. R 4. Homologous recombination replaced the ORF with a kanamycin cassette Recombinant chromosome 5'-flanking region Kan. R 3'-flanking region Where else is the homologous recombination machinery used?

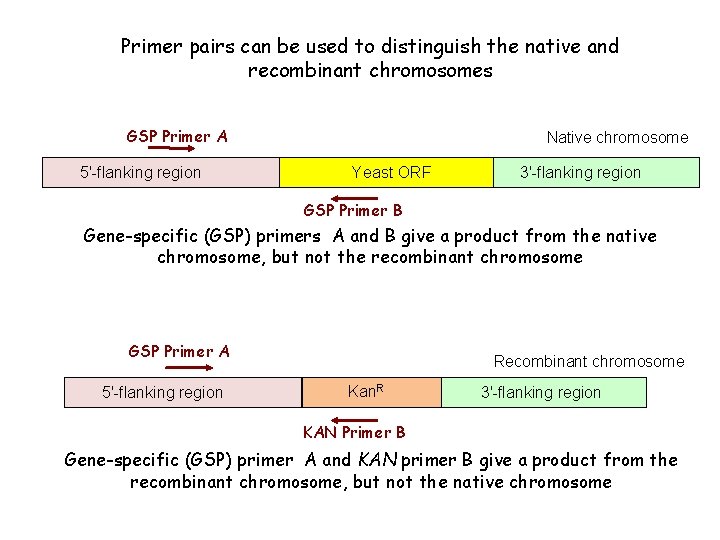
Primer pairs can be used to distinguish the native and recombinant chromosomes GSP Primer A 5'-flanking region Native chromosome Yeast ORF 3'-flanking region GSP Primer B Gene-specific (GSP) primers A and B give a product from the native chromosome, but not the recombinant chromosome GSP Primer A 5'-flanking region Recombinant chromosome Kan. R 3'-flanking region KAN Primer B Gene-specific (GSP) primer A and KAN primer B give a product from the recombinant chromosome, but not the native chromosome

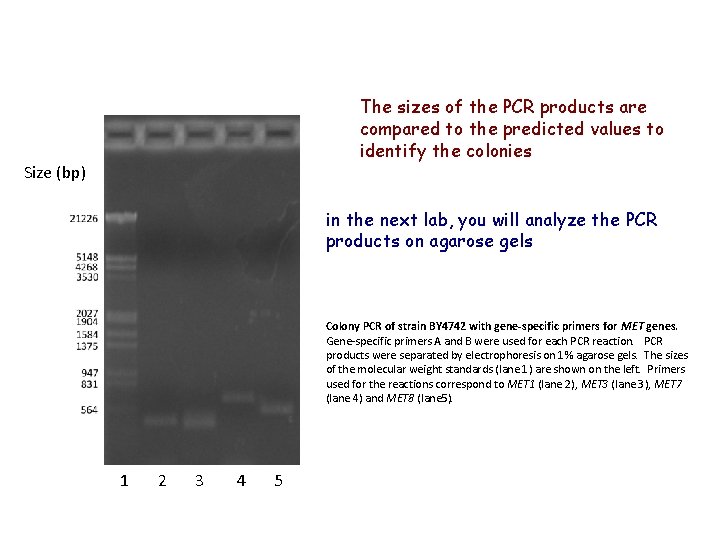
The sizes of the PCR products are compared to the predicted values to identify the colonies Size (bp) in the next lab, you will analyze the PCR products on agarose gels Colony PCR of strain BY 4742 with gene-specific primers for MET genes. Gene-specific primers A and B were used for each PCR reaction. PCR products were separated by electrophoresis on 1% agarose gels. The sizes of the molecular weight standards (lane 1) are shown on the left. Primers used for the reactions correspond to MET 1 (lane 2), MET 3 (lane 3), MET 7 (lane 4) and MET 8 (lane 5). 1 2 3 4 5