Year End Review Environmental Chemistry Environmental Chemistry n








































- Slides: 52

+ Year End Review Environmental Chemistry

+ Environmental Chemistry n Study of chemical processes in the environment. n Environmental chemists study which chemicals occur naturally in our environment, and in what concentrations. n Environmental chemistry is used by Environment Canada and other organizations around the world to identify sources of pollution and their potential impacts on the environment.

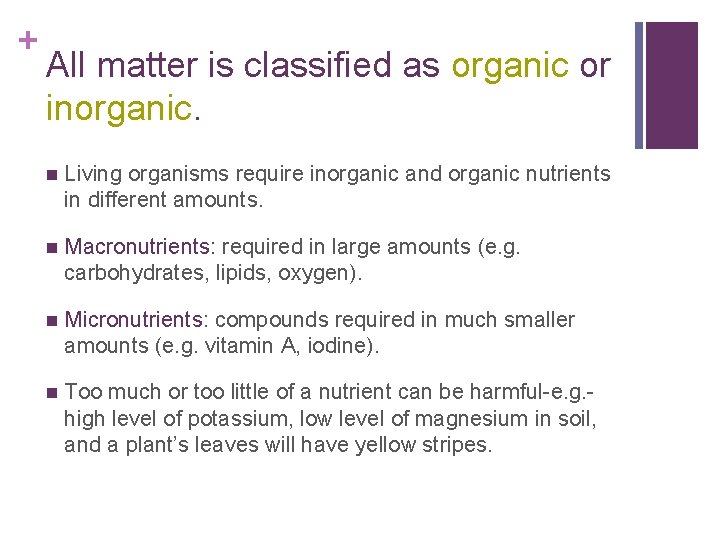
+ All matter is classified as organic or inorganic. n Living organisms require inorganic and organic nutrients in different amounts. n Macronutrients: required in large amounts (e. g. carbohydrates, lipids, oxygen). n Micronutrients: compounds required in much smaller amounts (e. g. vitamin A, iodine). n Too much or too little of a nutrient can be harmful-e. g. high level of potassium, low level of magnesium in soil, and a plant’s leaves will have yellow stripes.


+ Inorganic Elements: n. Magnesium: required for carrying out photosynthesis and maintaining metabolic reactions in animals.

+ Inorganic Elements: n. Potassium: required for stimulating protein production in plants and muscle contractions in animals.

+ Inorganic Elements: n. Calcium/Phosphorus: required for carrying out cell division in plants and for growing teeth and bone in animals.

+ Inorganic Elements: n. Nitrogen: required for building proteins. Remember-nitrogen fixing bacteria are found in the roots of some plants that help convert free nitrogen into a usable form for plants. Review Nitrogen Cycle.


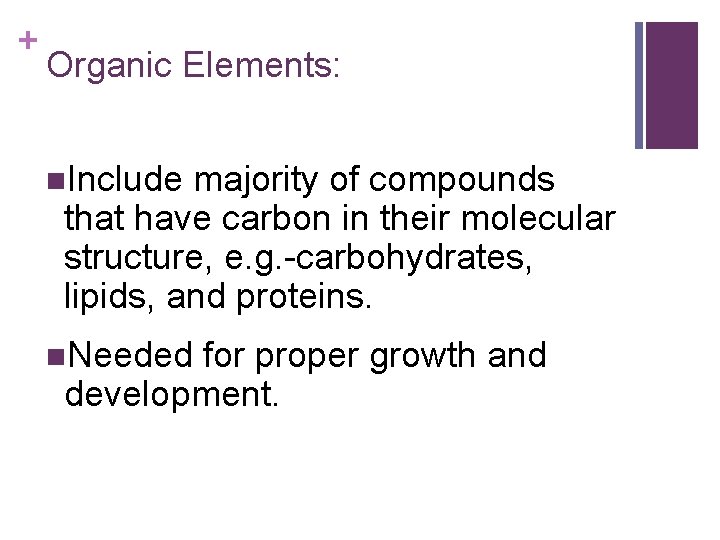
+ Organic Elements: n. Include majority of compounds that have carbon in their molecular structure, e. g. -carbohydrates, lipids, and proteins. n. Needed for proper growth and development.


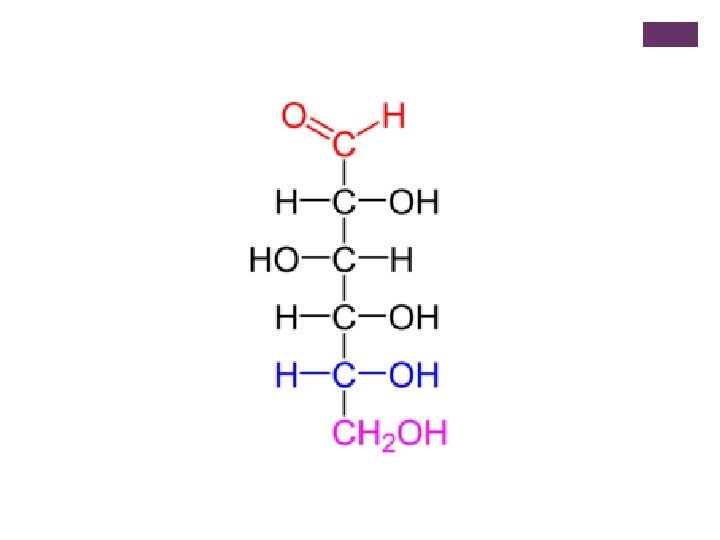
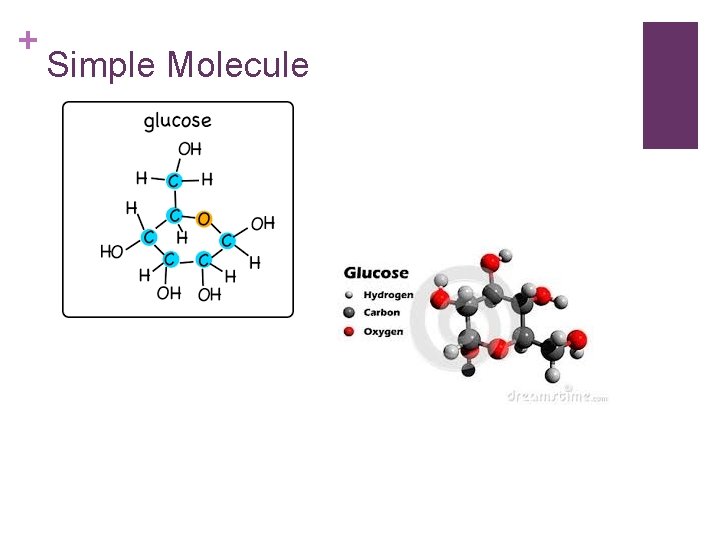
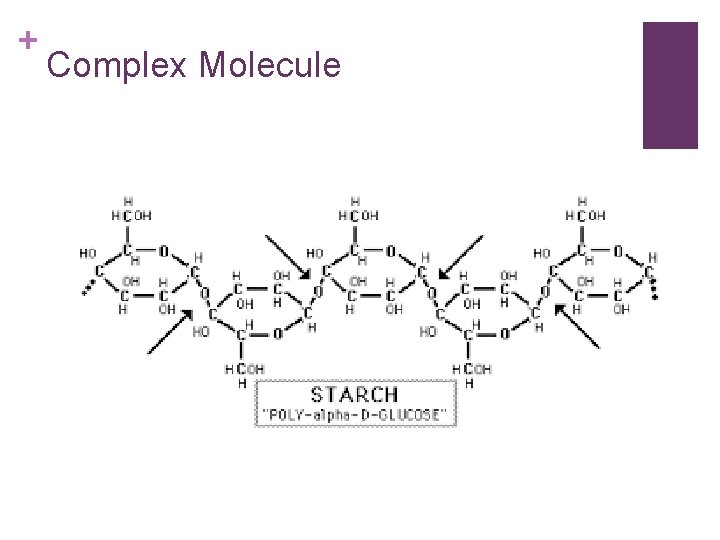
+ Organic Substances-synthesized by plants and animals include: n Carbohydrates: made up of carbon, hydrogen, and oxygen atoms. *most abundant food type that plants make for humans. n Can be simple molecules: glucose, fructose, and sucrose. n Or complex molecules: starch, cellulose, and glycogen. n Green plants synthesize glucose during photosynthesis. When we use the word synthesize this way, we mean: to make something new.

+ Simple Molecule

+ Complex Molecule

+ Organic Substances-synthesized by plants and animals include: n Lipids: made up of carbon, hydrogen, and oxygen atoms. n Fats, oils, and waxes produced by plants and animals are called lipids. n Fats: store energy from food; fat made up of fatty acids and glycerol. n Oils/waxes: protects the skin.

+ Organic Substances-synthesized by plants and animals include: n Proteins: made up of carbon, hydrogen, oxygen, and nitrogen atoms. n Proteins are necessary for the growth and repair of tissue, and they can be a source of energy. n Proteins are made up of amino acid molecules joined together and arranged in a specific sequence.

+ Organic Substances-synthesized by plants and animals include: n Nucleic Acids: Made up of a phosphate, simple sugar (ribose or deoxyribose), and nitrogencontaining bases. n Largest complex molecules found in living organisms, and pass on characteristics and control all cell activity. *responsible for heredity n All cells contain deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).

+ Chemicals introduced into the environment: n Happens due to natural processes and human activity. n Carbon Dioxide: introduced naturally during cellular respiration, and by human activity, such as driving a car, or burning coal. n The accumulation of unwanted waste material in the environment is called pollution.

+ Pollution is caused by: n Solid waste disposal n Waste water disposal n Combustion n Agricultural n Industrial activities processes n Chemicals that are introduced into the environment that cannot be broken down have long term effects on the environment.

+ Agricultural Chemicals introduced into the environment: n Fertilizers: contain nitrogen, phosphorus, potassium, and (sometimes) sulfur. n Important for crop growth, but overuse pollutes water and soil. n Can cause excess plant growth (bloom) which results in oxygen depletion when the plants decay.

+ Agricultural Chemicals introduced into the environment: n Pesticides: kill organisms that damage crops. n Herbicides: kill or control weeds. n Insecticides: n Fungicides: n All kill or control insecticides. kill fungi. of these chemicals pose a serious threat to the ecosystem.

+ Solid Waste Disposal n Plastic liners and clay are used in sanitary landfills to prevent chemicals from leaching (leaking) into the ground. n Some waste is too hazardous to be put into landfills and is burned in incinerators; burning garbage produces air pollution.

+ Wastewater Disposal n Sewage: wastewater collected from bathrooms, kitchens, and laundry rooms. n Contains dissolved and undissolved substances which can be harmful- rural areas- collected in septic tanks; urban areas- sent to sewage treatment plants. n Once the sewage has been broken down by bacteria or other chemical processes, the treated wastewater (called effluent) is released back into rivers, lakes, and oceans.

+ Biodegradeable n. In most sewage treatment processes, there is a stage at which the waste is broken down by bacteria; the fact that this waste can be broken down by bacteria indicates that the waste is biodegradeable.

+ Combustion n When fossil fuels (coal, petroleum, natural gas) are burned, they release large amounts of carbon dioxide and varying amounts of sulfur dioxide. n Sulfur dioxide combines with water in the atmosphere, and causes acid rain or snow. n Combustion in vehicles is a major source of ground level ozone which causes breathing problems, especially in people with respiratory diseases.

+ Industrial Processes n Harmful wastes are introduced into the environment during various industrial processes, even in a chocolate factory n E. g. Processing of crude oil- crude oil is a mixture of many compounds with high degrees of toxicity; it is important to minimize the release of these compounds into the air when producing petroleum.

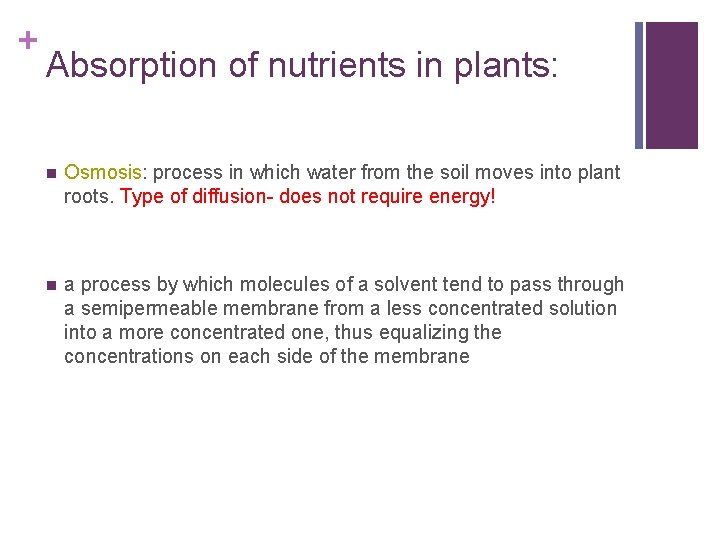
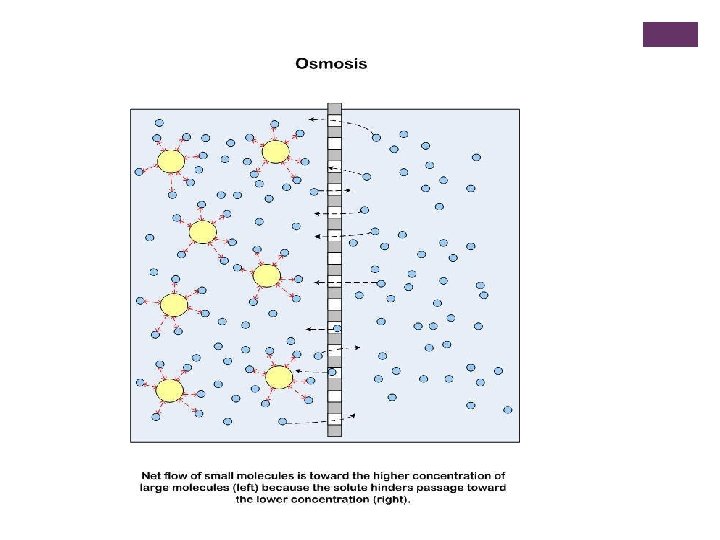
+ Absorption of nutrients in plants: n Osmosis: process in which water from the soil moves into plant roots. Type of diffusion- does not require energy! n a process by which molecules of a solvent tend to pass through a semipermeable membrane from a less concentrated solution into a more concentrated one, thus equalizing the concentrations on each side of the membrane


+ Active Transport n. Movement of a material from an area of low concentration to an area of high concentration. n. Unlike osmosis and diffusion, this process requires energy!

+ Ingestion: n Process by which plants and animals take in energy. Foods is broken down mechanically and chemically into simpler substances which can be used by the body. n Hydrolysis: Process in which large organic molecules (like sugar) are broken down with water. n Nutrients are then small enough they can be absorbed into the blood stream.

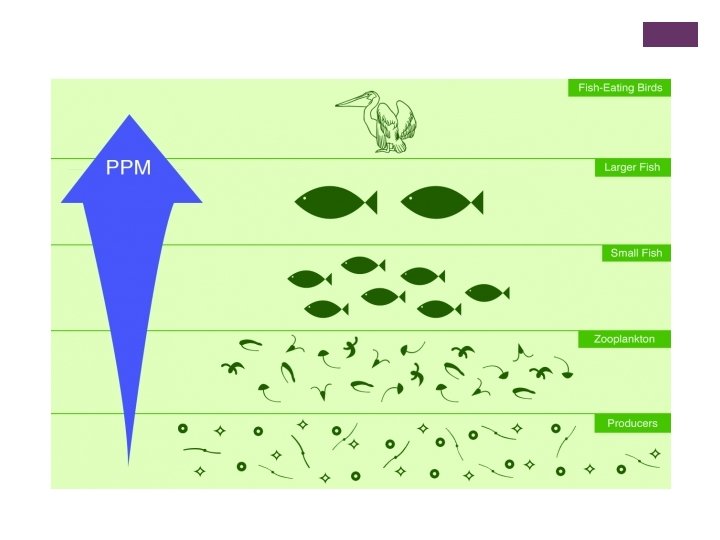
+ Bioaccumulation: n Even if there are low levels of a toxin in the environment, chemical compounds can accumulate in a body over time- especially when it is a compound the body cannot easily break down. n This n The can make the organism sick, or even kill it. process of low levels of chemicals building up in the bodies of living organisms is called bioaccumulation.

+ Biomagnification: n This is the “passing on” of toxins when an organism/animal is eaten. n Toxins increase at each level of the food chain (trophic levels) because many individual organisms must be eaten by the larger predator to support life. n E. g. Imagine that a mouse has “a bit” of toxins in its body, but that a fox has to eat “many” mice to survive. The fox would have “magnified” the amount of toxins in its tissues by taking in all the toxins in the individual mice. The small amount of toxins in each mouse may not have harmed each individual mouse, but all the accumulated toxin will harm the fox.



+ Safe Release of Substances into the Environment: n Remember that humans are trying to improve their environmental practices by developing new technologies which cause less harm to the environment, and by choosing alternative methods: n This includes energy efficient engines that burn less fuel, catalytic converters for muffler systems that remove pollutants from vehicle exhaust, carpooling, bussing, etc.

+ Substrate: n Any surface on which a plant or animal lives or moves. n Some animals get their nutrition from their substrate: e. g. forest floor is made up of decaying vegetation. Soil provides a substrate for a large number of decomposers such as bacteria, fungi, algae, and worms.

+ Biological Indicators n Using organisms to determine the health of an environment/whether or not the environment is polluted/suffering. n E. g. Invertebrate aquatic organisms found in a water habitat are an excellent indicator of water quality (mosquito larvae, leech, stonefly larva, midge larva, caddisfly larva, mayfly larva) n Factors such as water PH, and dissolved oxygen affect the kind of organisms found in water habitats.

+ Air Quality: n Is constantly monitored and good air quality should be maintained. n Collecting data about chemicals in the air provides information about immediate and long term trends. n There is concern about emissions of sulfur dioxide, nitrogen oxide, carbon dioxide, and chlorofluorocarbons in the atmosphere and the effect of these chemicals on the environment.

+ Chemical Factors: n Chemical concentrations indicate the quality of water and may affect the distribution of living organisms in an ecosystem. n Natural rain water has a p. H of 5; precipitation with a p. H lower than 5. 6 is called acid rain. Few organisms are able to survive in an acidic environment. n Elements such a s mercury, copper, lead, zinc, cadmium, and nickel are heavy metals. Heavy metals that enter the environment can accumulate in animals and cause illness, deformities, and death.

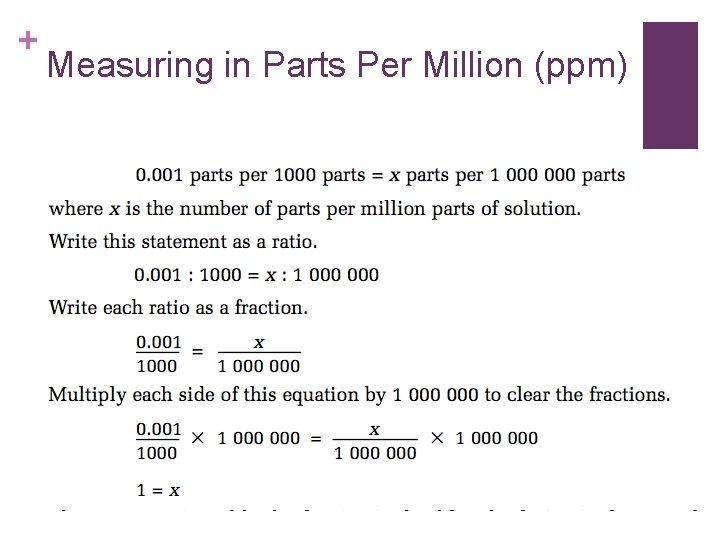
+ Measuring in Parts Per Million (ppm)

+ A solution is made: n. By dissolving 3 m. L of food colouring in 997 m. L of water. n. In this solution the concentration of food colouring is?

+ n 3 000 ppm

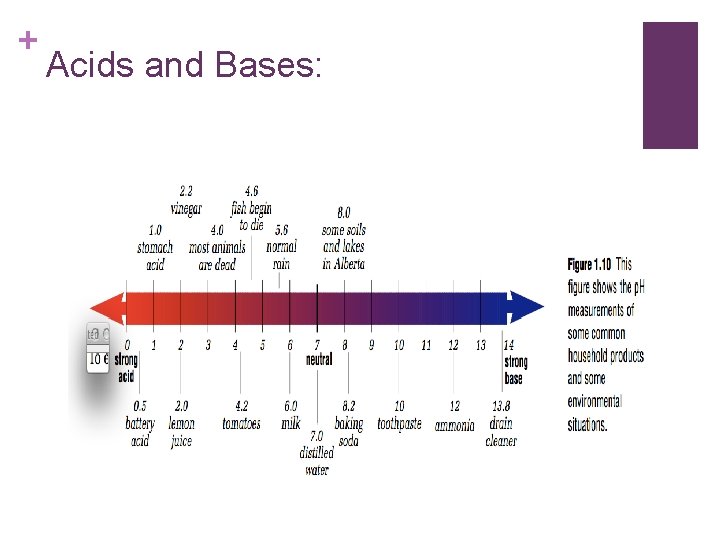
+ Acids and Bases:

+ Neutral Substances: n. Are neither acidic or basic and have a p. H of 7. Distilled water and blood are neutral substances.

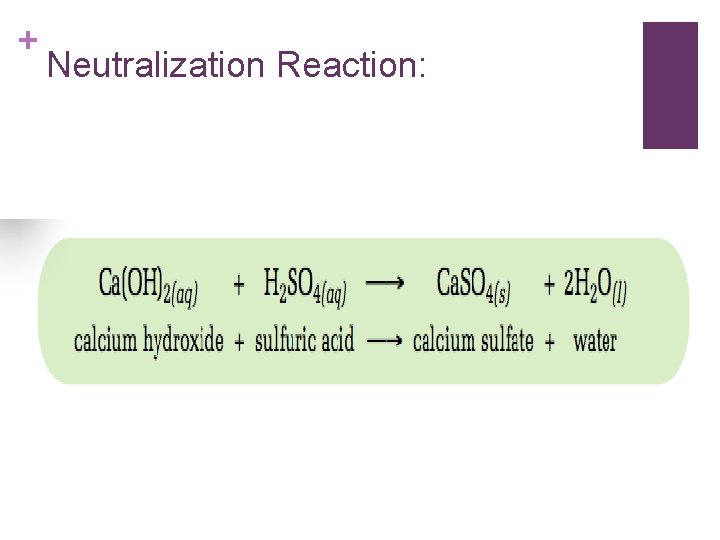
+ Neutralization: n. A neutralization reaction is a reaction between and acid and a base. When an acid reacts with a base, salt and water are produced.

+ Neutralization Reaction:

+ Acid rain: n. The effects of acid rain can be reduced by treating lakes with lime (calcium oxide). Lime is dissolved into the water and neutralizes the acid present in the lake water.

+ Transport through air, soil, and water: n Occurs in 3 stages: n Release of chemicals from the source n Dispersion of chemicals into the atmosphere (influenced by factors such as properties of chemicals, speed and direction of wind, whether or not they get into groundwater or remain on surface water). n Deposition of chemicals in the soil or water (permeable soil has larger pores and it is easier for pollutants to flow and spread through permeable soil).

+ Biodegradation: n When the concentration of pollutants in the environment is decreased naturally by certain organisms such as bacteria, fungi and earthworms in the soil. n When this process needs oxygen, it is referred to as aerobic biodegradation. n When this process does not need oxygen, the bacteria that decompose organic material through anaerobic biodegradation.

+ Bioreactors: n. Have been built to increase the rate of biodegradation. n. A sewage treatment plant has bioreactors to speed up the decomposition of sewage.

+ Median lethal dose, LD 50 n Toxicity describes how poisonous a substance is. n The LD 50 is the dose of a toxic substance required to kill half the members of a tested population after a specified test duration (length of time). n E. g. The LD 50 dose in of DDT in rats is 87 mg/kg. Half the rats in a population would die if given a dosage of 87 mg/kg of DDT

+ WHMIS n WHMIS labels on containers specify the type of hazardous chemicals in the containers. n Material Safety Data Sheets (MSDS) give detailed descriptions of chemical products. n They describe the precautions that should be taken while handling, storing, transporting or disposing of that product- review in text-e. g. Keep chemicals out of the reach of children.
 Twinkl year 6 leavers poem
Twinkl year 6 leavers poem End of year review objectives
End of year review objectives Grade 10 accounting year end adjustments
Grade 10 accounting year end adjustments When is amazon's fiscal year end
When is amazon's fiscal year end Greenville bar cle
Greenville bar cle End of the year poetry
End of the year poetry Year end planning
Year end planning Accounting adjustments grade 10
Accounting adjustments grade 10 End of year school reports 2021
End of year school reports 2021 Reception end of year expectations
Reception end of year expectations Stroke volume definition
Stroke volume definition Edv and preload
Edv and preload Front end and back end in compiler design
Front end and back end in compiler design Front end phases of compiler
Front end phases of compiler End zu end descendorektostomie
End zu end descendorektostomie End-to-end wireframe parsing
End-to-end wireframe parsing End to end argument in system design
End to end argument in system design End to end accounting life cycle tasks
End to end accounting life cycle tasks End to end delay
End to end delay End to end delay
End to end delay End to end
End to end Comet transformer
Comet transformer End-to-end procurement life cycle
End-to-end procurement life cycle 25 year environment plan summary
25 year environment plan summary Mid year budget review
Mid year budget review Amway products catalogue 2013
Amway products catalogue 2013 Mid year budget review
Mid year budget review Environmental science final study guide
Environmental science final study guide Hbs eoc study guide
Hbs eoc study guide Wireless health
Wireless health Environmental science chapter 11
Environmental science chapter 11 Chapter 13 review environmental science
Chapter 13 review environmental science Chapter 8 environmental science
Chapter 8 environmental science Environmental science chapter 2 review answers
Environmental science chapter 2 review answers Three levels of biodiversity
Three levels of biodiversity Chapter review motion part a vocabulary review answer key
Chapter review motion part a vocabulary review answer key Ap gov review final exam review
Ap gov review final exam review Nader amin-salehi
Nader amin-salehi Traditional and systematic review venn diagram
Traditional and systematic review venn diagram Narrative review vs systematic review
Narrative review vs systematic review Elements of environmental chemistry
Elements of environmental chemistry Environmental chemistry science olympiad
Environmental chemistry science olympiad Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Love formula in chemistry
Love formula in chemistry Chapter 14 acids and bases
Chapter 14 acids and bases Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Chapter 12 review solutions section 1
Chapter 12 review solutions section 1 Grade 9 and 10 chemistry review
Grade 9 and 10 chemistry review Geometry sol review packet
Geometry sol review packet Chemistry unit review answer key
Chemistry unit review answer key Kuhinjska sol formula
Kuhinjska sol formula Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions