Year 9 Science Matter Atoms and The Periodic


- Slides: 16

Year 9 Science: Matter Atoms and The Periodic Table

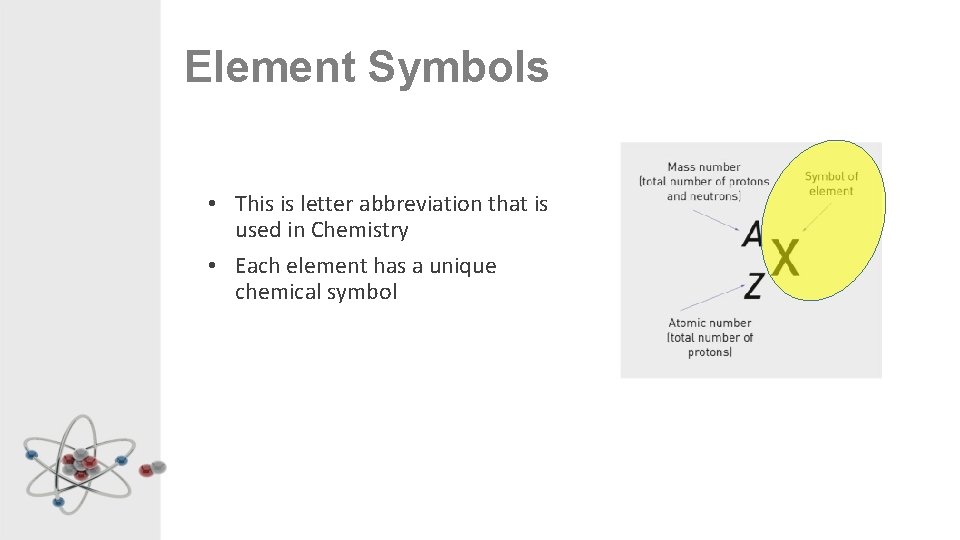
Learning Intentions Learning intention: To understand that atoms have mass relative to the number of protons and neutrons they contain. Success Criteria: • Identify the relative mass of protons, neutrons and electrons. • Calculate the mass number of the first 20 elements of the periodic table. • Identify the conventional representation of an element (symbol, atomic mass and atomic number).


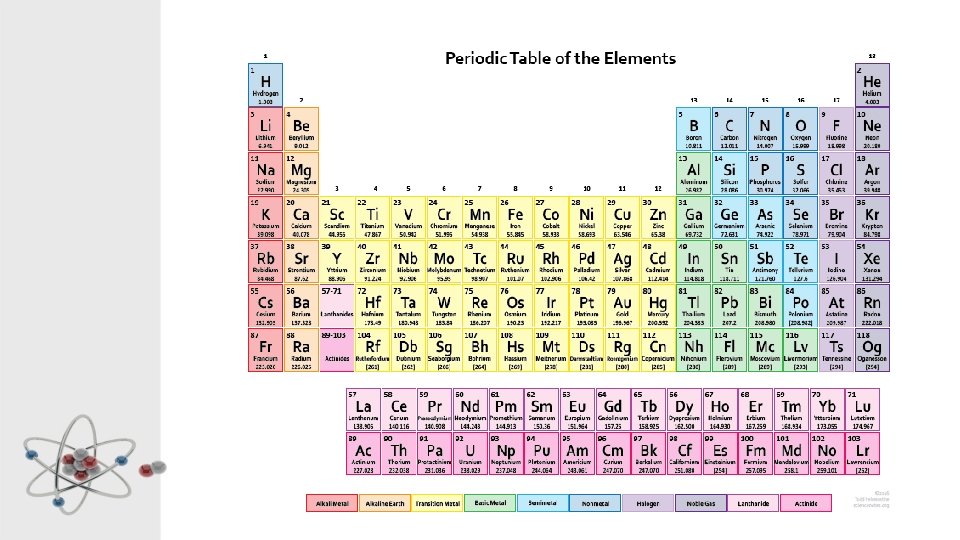
The Periodic Table • Each element is made of a different type of atom • The Periodic Table displays and organises all the known elements • Each type of atom has it’s own square (or rectangle)

Elements

Element Symbols • This is letter abbreviation that is used in Chemistry • Each element has a unique chemical symbol

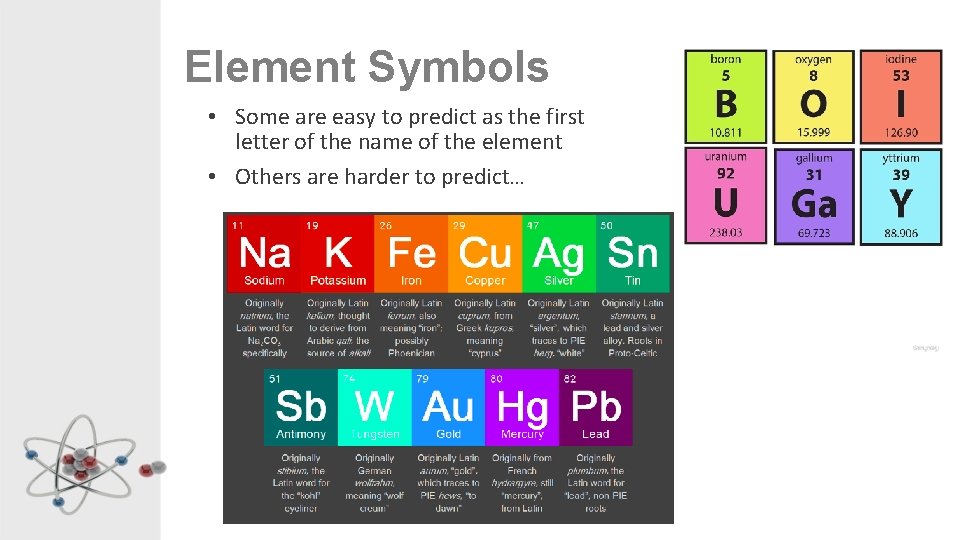
Element Symbols • Some are easy to predict as the first letter of the name of the element • Others are harder to predict…

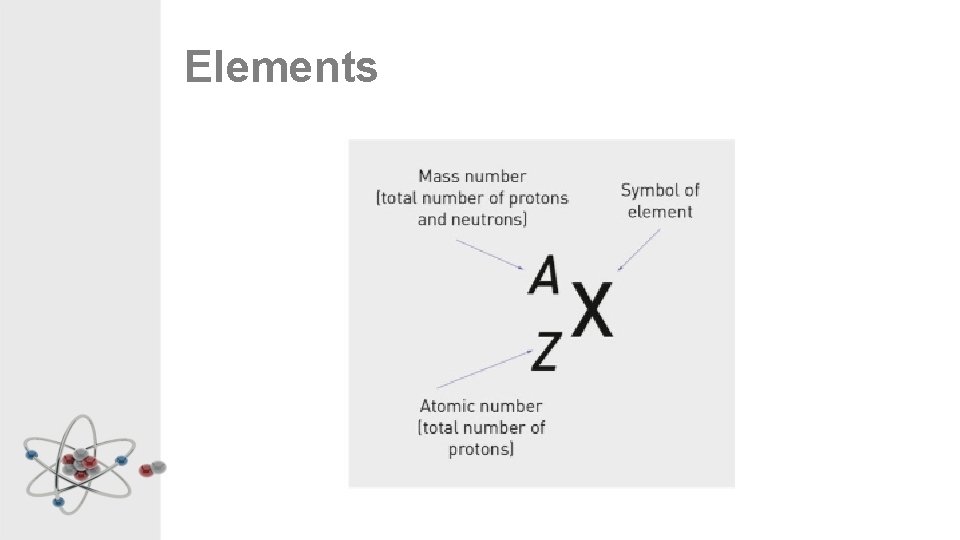
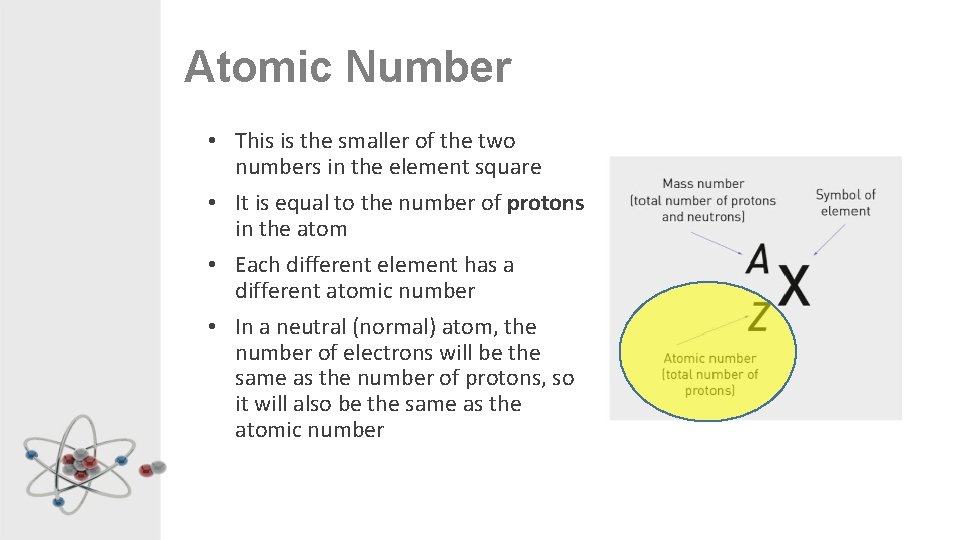
Atomic Number • This is the smaller of the two numbers in the element square • It is equal to the number of protons in the atom • Each different element has a different atomic number • In a neutral (normal) atom, the number of electrons will be the same as the number of protons, so it will also be the same as the atomic number

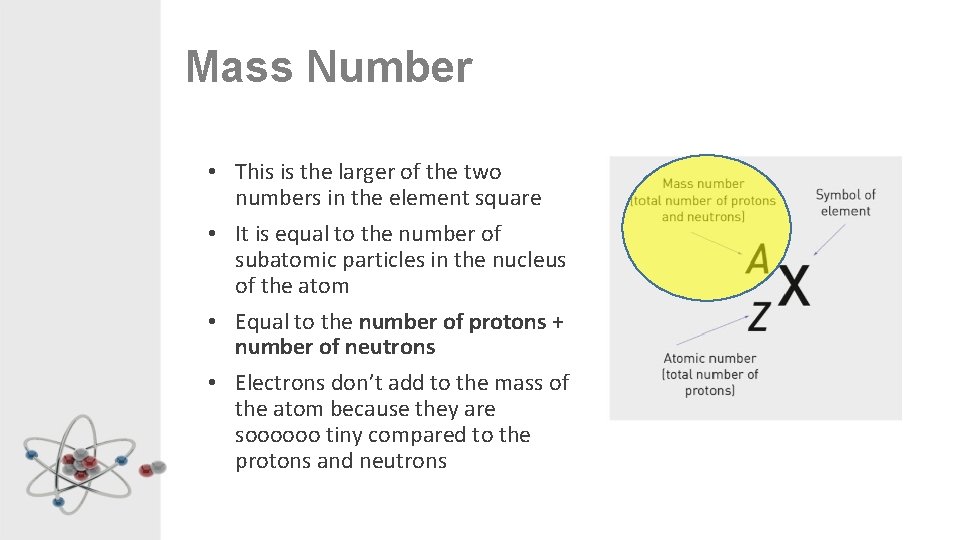
Mass Number • This is the larger of the two numbers in the element square • It is equal to the number of subatomic particles in the nucleus of the atom • Equal to the number of protons + number of neutrons • Electrons don’t add to the mass of the atom because they are soooooo tiny compared to the protons and neutrons

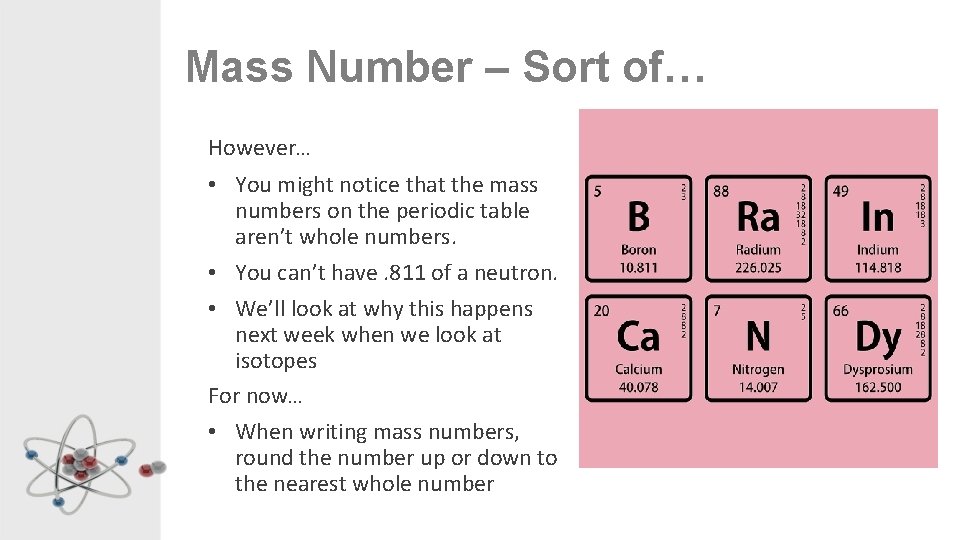
Mass Number – Sort of… However… • You might notice that the mass numbers on the periodic table aren’t whole numbers. • You can’t have. 811 of a neutron. • We’ll look at why this happens next week when we look at isotopes For now… • When writing mass numbers, round the number up or down to the nearest whole number

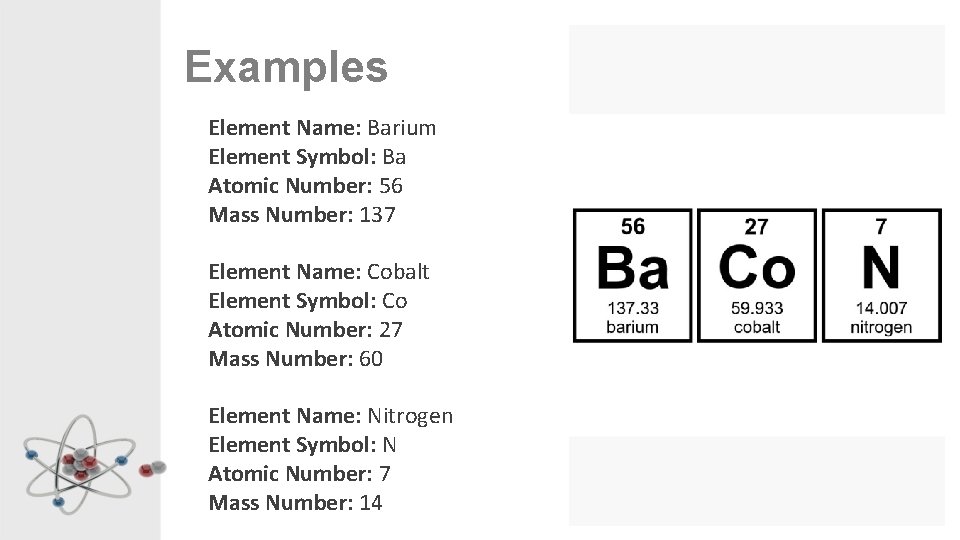
Examples Element Name: Barium Element Symbol: Ba Atomic Number: 56 Mass Number: 137 Element Name: Cobalt Element Symbol: Co Atomic Number: 27 Mass Number: 60 Element Name: Nitrogen Element Symbol: N Atomic Number: 7 Mass Number: 14

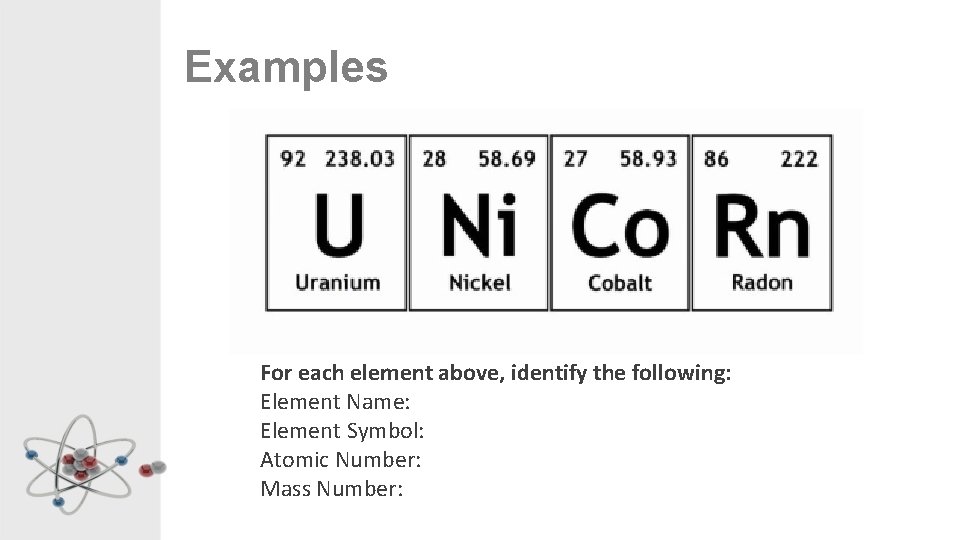
Examples For each element above, identify the following: Element Name: Element Symbol: Atomic Number: Mass Number:

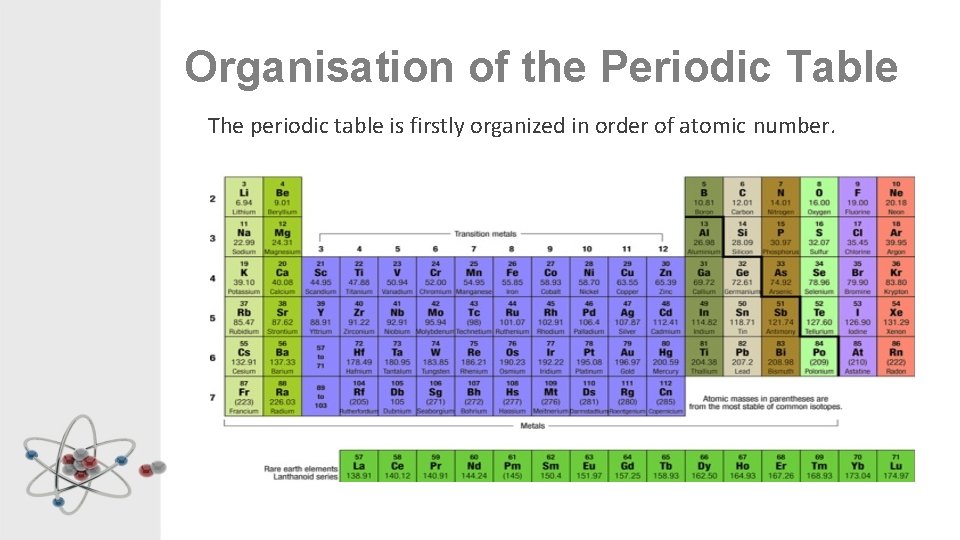
Organisation of the Periodic Table The periodic table is firstly organized in order of atomic number.

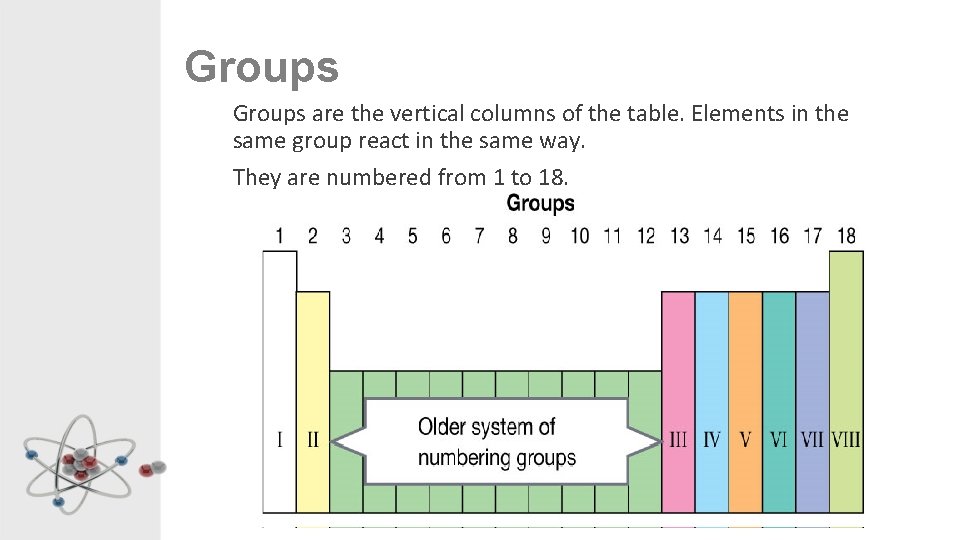
Groups are the vertical columns of the table. Elements in the same group react in the same way. They are numbered from 1 to 18.

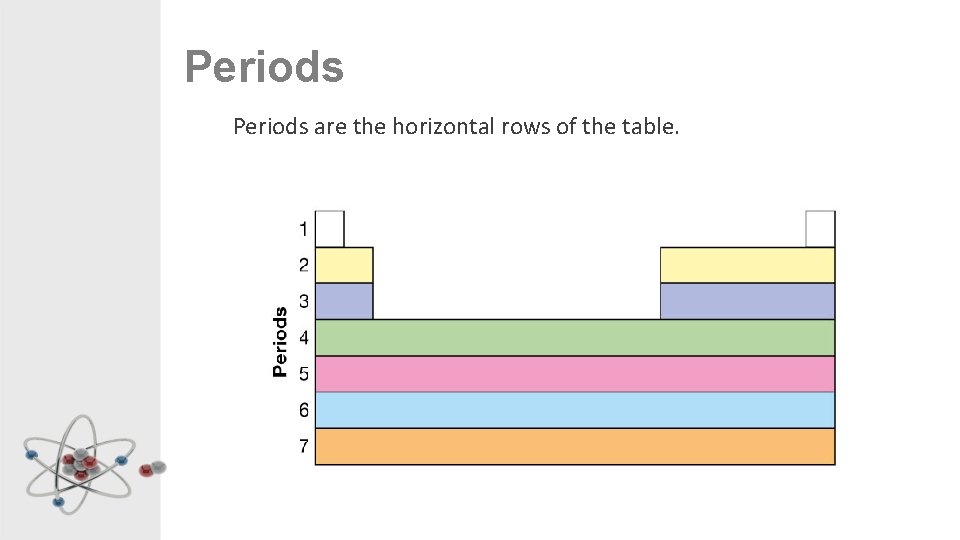
Periods are the horizontal rows of the table.

Activities • • • Complete Notes Worksheet: Atomic Symbols and Numbers Worksheet: Atomic Numbers and Mass Numbers Check Your Learning 5. 3 Q 2 -6 Study Strip