Year 2 pharmacology Haemostasis thrombosis NSAIDs and atherosclerosis

Year 2 pharmacology: Haemostasis, thrombosis, NSAIDs and atherosclerosis Ailbe

My guide to pharmacology • Pharmacology makes up most of LCRS 1 (a long with endocrinology & RDA) • I would recommend spending a disproportionally small amount of time revising pharm, as the smaller topics like RDA, Musc, Psychology and the MCD topics can be where you lose a lot of marks (the questions are hard!) • Tips: o Learn the ”basics” from the first few lectures, such as the principles of pharmacodynamics and pharmacokinetics o Make a drug list of all the drugs, how they work, how they are cleared from the body, and side effects if possible o LEARN THIS LIST!! o Don’t get bogged down revising pharmacology, you are more likely to get easy pharm questions than easy psychology and RDA questions

My guide to pharmacology • The stuff in this lecture rarely comes up in the exam, maybe 2 -3 questions a year • Very unlikely to be an SAQ

Haemostasis and thrombosis
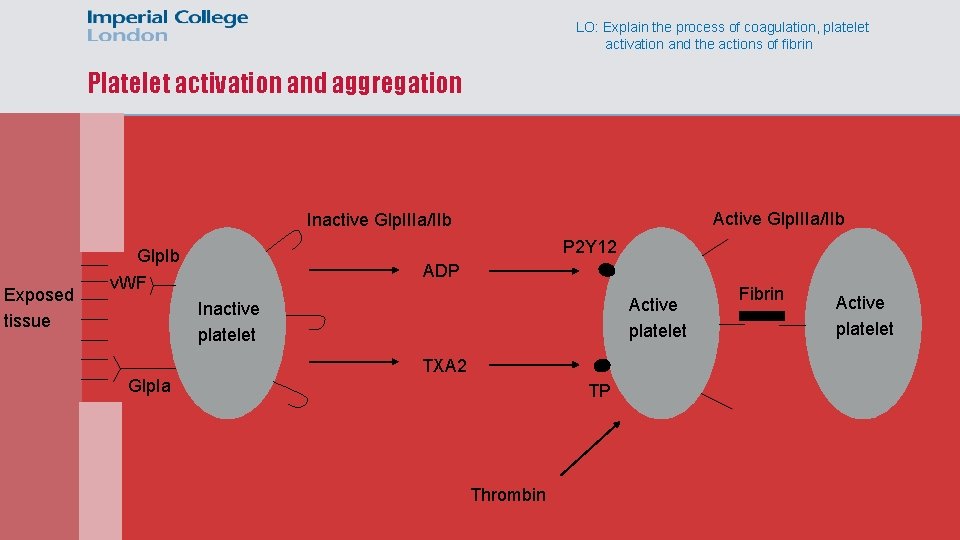
LO: Explain the process of coagulation, platelet activation and the actions of fibrin Platelet activation and aggregation Active Glp. IIIa/IIb Inactive Glp. IIIa/IIb Exposed tissue P 2 Y 12 Glp. Ib v. WF ADP Active platelet Inactive platelet TXA 2 Glp. Ia TP Thrombin Fibrin Active platelet
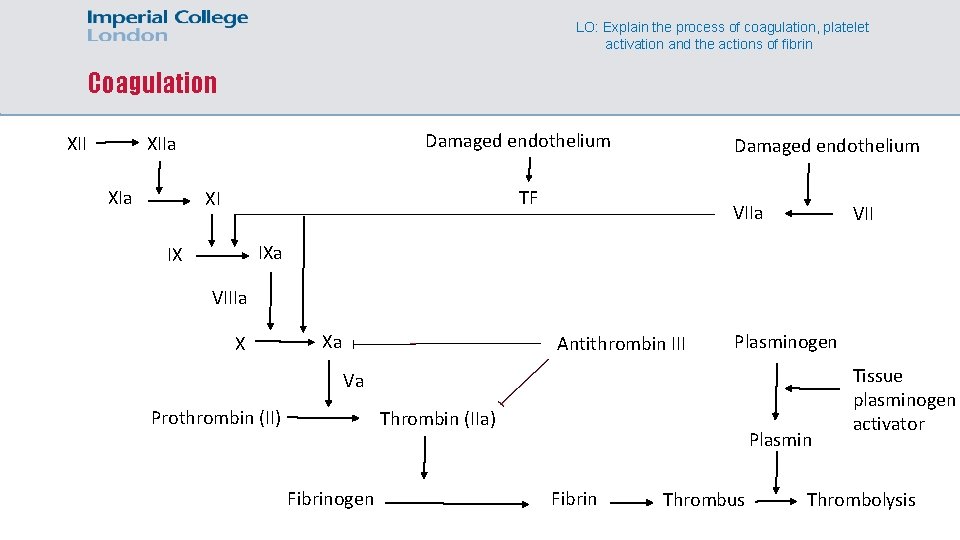
LO: Explain the process of coagulation, platelet activation and the actions of fibrin Coagulation XII Damaged endothelium XIIa XIa Damaged endothelium TF XI VIIa VII IXa IX VIIIa X Xa Antithrombin III Plasminogen Va Prothrombin (II) Thrombin (IIa) Fibrinogen Plasmin Fibrin Thrombus Tissue plasminogen activator Thrombolysis

LO: Explain the process of coagulation, platelet activation and the actions of fibrin Platelet amplification • Thrombin generated from the coagulation cascade can also activate platelets • This amplifies the formation of the thrombus
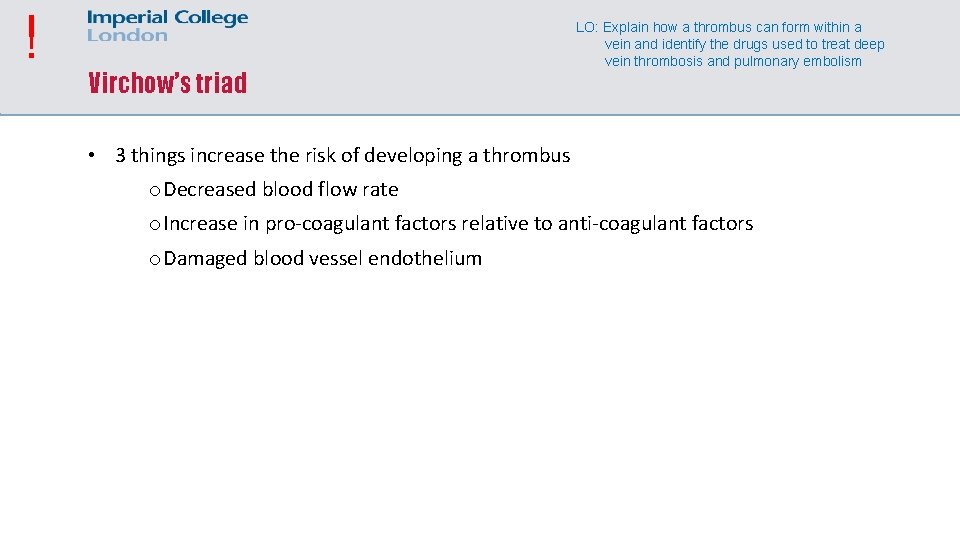
! Virchow’s triad LO: Explain how a thrombus can form within a vein and identify the drugs used to treat deep vein thrombosis and pulmonary embolism • 3 things increase the risk of developing a thrombus o Decreased blood flow rate o Increase in pro-coagulant factors relative to anti-coagulant factors o Damaged blood vessel endothelium

! DVT and PE LO: Explain how a thrombus can form within a vein and identify the drugs used to treat deep vein thrombosis and pulmonary embolism • Deep veins in the leg are prone to blood stasis, and therefore an increased risk of thrombus formation. Risk factors include surgery, prolonged immobility and obesity • Embolisation of the thrombus can lead to it travelling to the heart, where it can block a pulmonary artery, leading to PE

! DVT and PE LO: Explain how a thrombus can form within a vein and identify the drugs used to treat deep vein thrombosis and pulmonary embolism • DVTs are treated with heparin initially • Warfarin is given at the same time, but warfarin takes time to increase the INR (Warfarin has a paradoxical pro-thrombotic effect in the first few days due to inhibition of protein C and S, which usually inhibit factors 5 and 8, and thus inhibit thrombin formation. Therefore warfarin initially increases thrombin formation) • Direct oral anticoagulants such as dabigatran or rivaroxaban can be used instead of warfarin • PEs are treated the same way unless the patient is at severe risk of a cardiac arrest, in which case they need thromboylysis with a 50 mg bolus of a t. PA such as alteplase
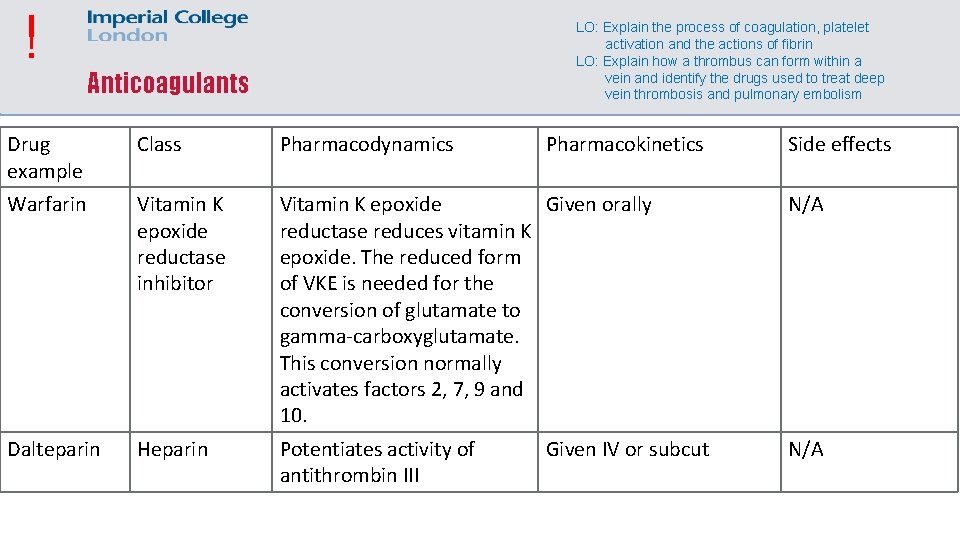
! LO: Explain the process of coagulation, platelet activation and the actions of fibrin LO: Explain how a thrombus can form within a vein and identify the drugs used to treat deep vein thrombosis and pulmonary embolism Anticoagulants Drug example Class Pharmacodynamics Pharmacokinetics Warfarin Vitamin K epoxide reductase inhibitor Vitamin K epoxide Given orally reductase reduces vitamin K epoxide. The reduced form of VKE is needed for the conversion of glutamate to gamma-carboxyglutamate. This conversion normally activates factors 2, 7, 9 and 10. N/A Dalteparin Heparin Potentiates activity of antithrombin III N/A Given IV or subcut Side effects
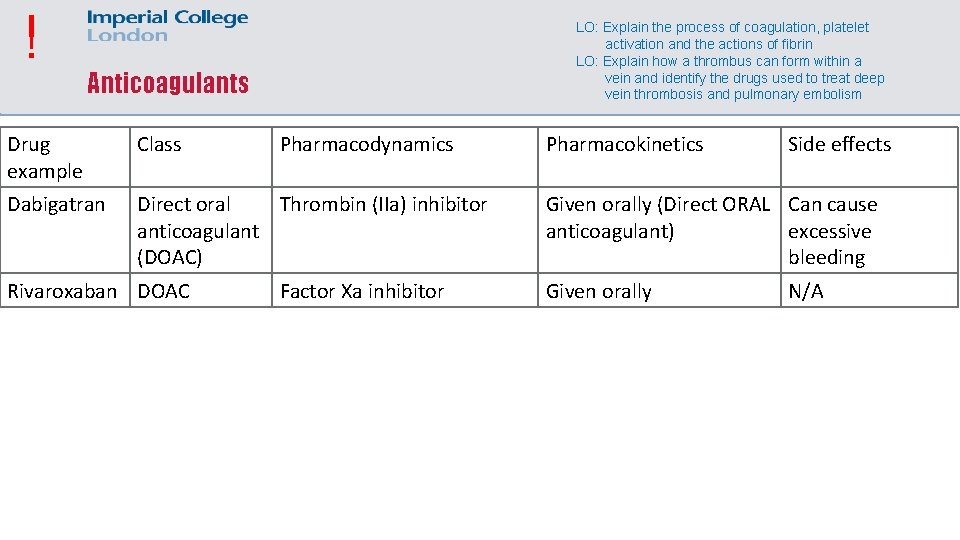
! LO: Explain the process of coagulation, platelet activation and the actions of fibrin LO: Explain how a thrombus can form within a vein and identify the drugs used to treat deep vein thrombosis and pulmonary embolism Anticoagulants Drug example Class Dabigatran Direct oral Thrombin (IIa) inhibitor anticoagulant (DOAC) Rivaroxaban DOAC Pharmacodynamics Factor Xa inhibitor Pharmacokinetics Side effects Given orally (Direct ORAL Can cause anticoagulant) excessive bleeding Given orally N/A

LO: Explain the process of coagulation, platelet activation and the actions of fibrin Acute coronary syndrome • NSTEMI – caused by a partially occluded coronary artery • Treated with aspirin and clopidogrel as well as heparin to reduce DVT risk • STEMI – caused by a fully occluded coronary artery • Treated with aspirin and clopidogrel and thrombolysis (if within 12 hours of onset) • ACS is caused by thrombus formation following damage to the vascular endothelium due to atheroma formation • Therefore antiplatelets are used to stop the thrombus growing any larger
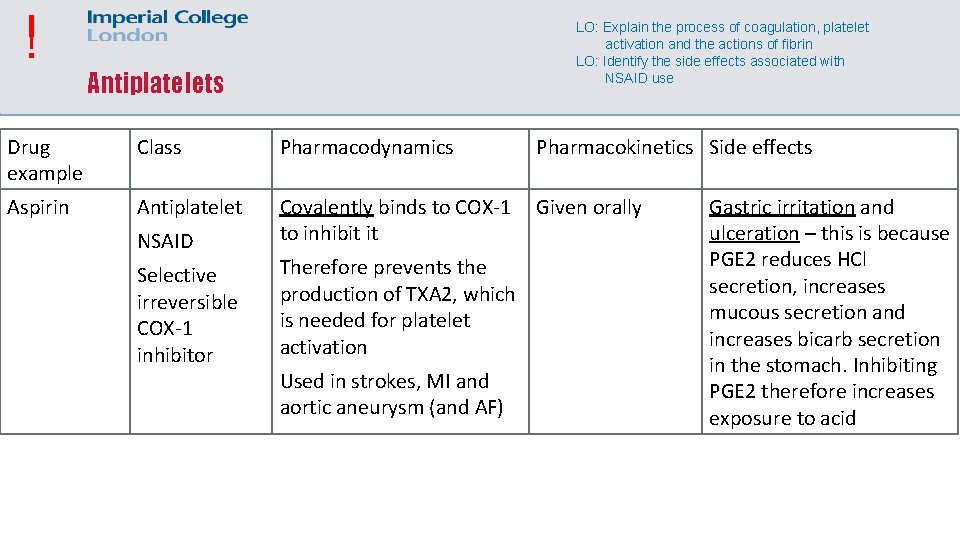
! LO: Explain the process of coagulation, platelet activation and the actions of fibrin LO: Identify the side effects associated with NSAID use Antiplatelets Drug example Class Pharmacodynamics Pharmacokinetics Side effects Aspirin Antiplatelet Covalently binds to COX-1 to inhibit it Given orally NSAID Selective irreversible COX-1 inhibitor Therefore prevents the production of TXA 2, which is needed for platelet activation Used in strokes, MI and aortic aneurysm (and AF) Gastric irritation and ulceration – this is because PGE 2 reduces HCl secretion, increases mucous secretion and increases bicarb secretion in the stomach. Inhibiting PGE 2 therefore increases exposure to acid
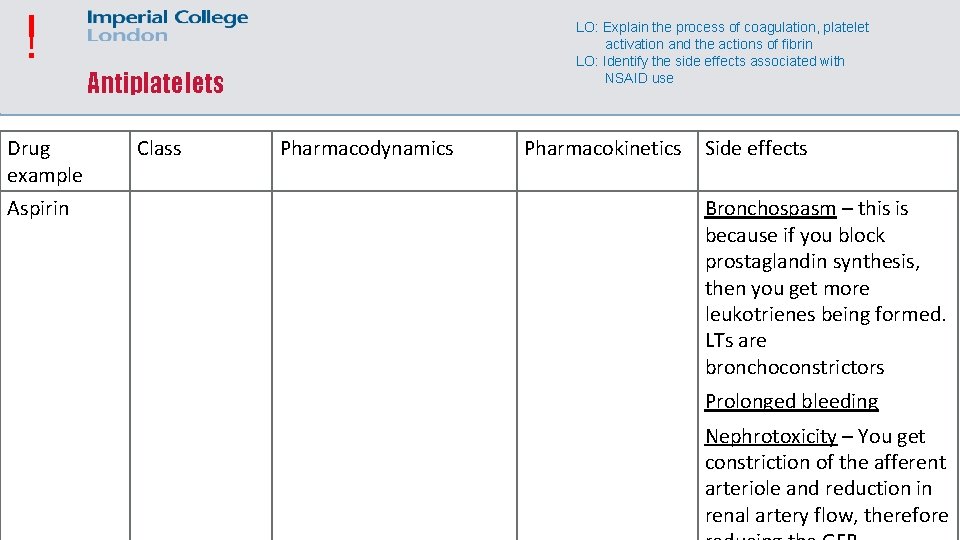
! Drug example Aspirin LO: Explain the process of coagulation, platelet activation and the actions of fibrin LO: Identify the side effects associated with NSAID use Antiplatelets Class Pharmacodynamics Pharmacokinetics Side effects Bronchospasm – this is because if you block prostaglandin synthesis, then you get more leukotrienes being formed. LTs are bronchoconstrictors Prolonged bleeding Nephrotoxicity – You get constriction of the afferent arteriole and reduction in renal artery flow, therefore
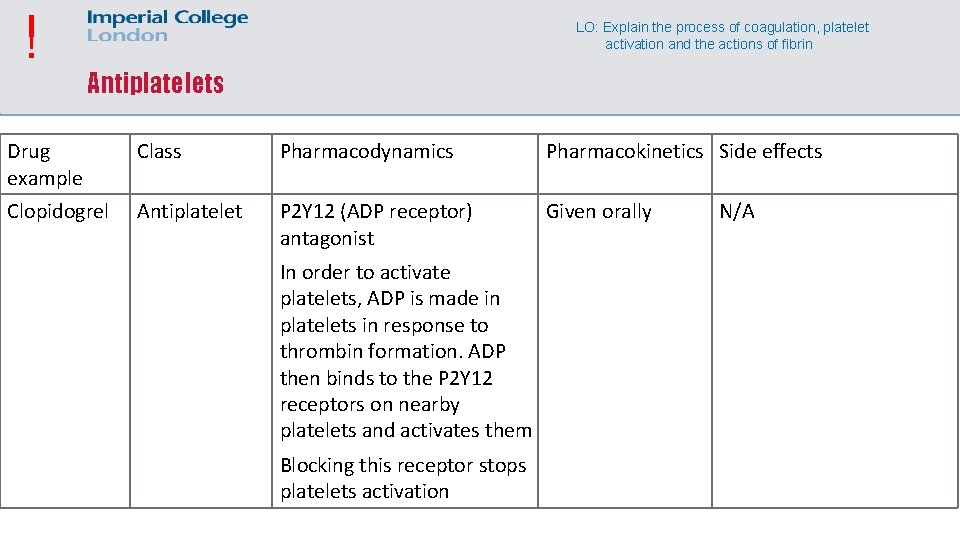
! LO: Explain the process of coagulation, platelet activation and the actions of fibrin Antiplatelets Drug example Class Pharmacodynamics Pharmacokinetics Side effects Clopidogrel Antiplatelet P 2 Y 12 (ADP receptor) antagonist Given orally In order to activate platelets, ADP is made in platelets in response to thrombin formation. ADP then binds to the P 2 Y 12 receptors on nearby platelets and activates them Blocking this receptor stops platelets activation N/A
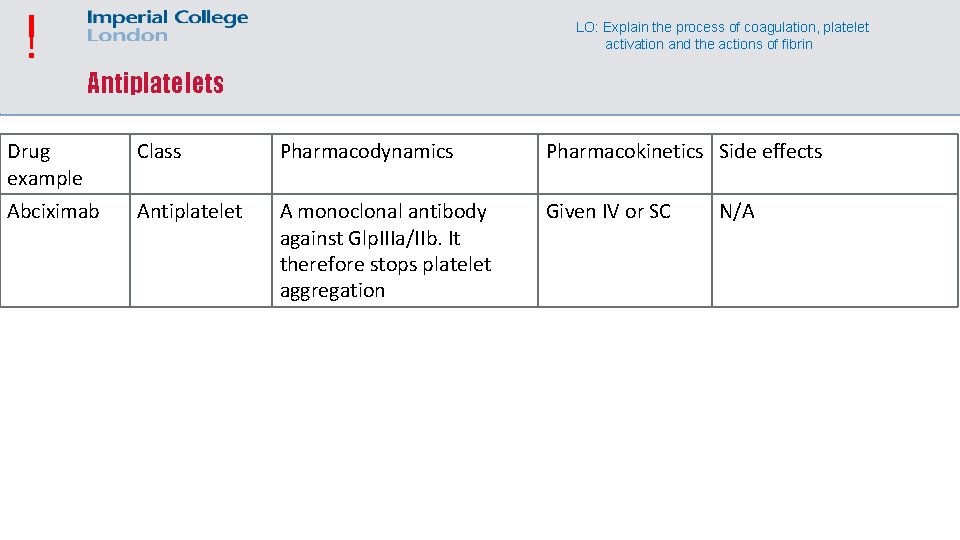
! LO: Explain the process of coagulation, platelet activation and the actions of fibrin Antiplatelets Drug example Class Pharmacodynamics Pharmacokinetics Side effects Abciximab Antiplatelet A monoclonal antibody against Glp. IIIa/IIb. It therefore stops platelet aggregation Given IV or SC N/A

LO: Explain the process of coagulation, platelet activation and the actions of fibrin Ischaemic strokes • Caused by thrombosis in one of the arteries supplying the brain or embolism from somewhere in the body (e. g. in AF) • Thrombolysis with alteplase may be considered if within 4. 5 hours of onset • If greater than 4. 5 hours of onset, then aspirin and clopidogrel are given as well as heparin if the patient is at a high risk of more emboli
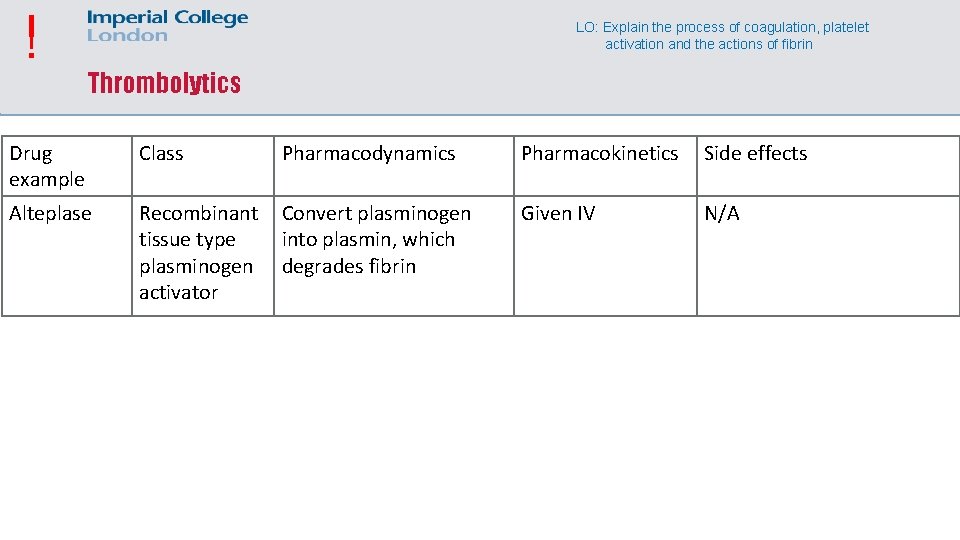
! LO: Explain the process of coagulation, platelet activation and the actions of fibrin Thrombolytics Drug example Class Pharmacodynamics Pharmacokinetics Side effects Alteplase Recombinant tissue type plasminogen activator Convert plasminogen into plasmin, which degrades fibrin Given IV N/A

LO: Explain the process of coagulation, platelet activation and the actions of fibrin GENERAL RULE Arterial disease (strokes and heart attacks) is treated with antiplatelets Venous/stasis disease (AF, DVT and PE) is treated with anticoagulants However there are exceptions: Anticoagulants are given in stroke and ACS if the patient has AF or is being kept in hospital and so is at risk of DVT Antiplatelets are still often given for stroke prevention in people with AF, despite anticoagulants being superior at preventing stroke compared to aspirin in AF

Which drug directly inhibits factor 10 a? A) Warfarin sodium B) Dalterparin C) Edoxaban D) Dabigatran E) Alteplase

What drug would you give to someone with DVT? A) Glp IIIa/IIb inhibitor B) Direct thrombin inhibitor C) Factor Xa inhibitor D) Clopidogrel E) Alteplase
Which of these drugs is given intravenously? A) Alteplase B) Warfarin C) Dabigatran D) Rivaroxaban E) Aspirin

NSAIDs
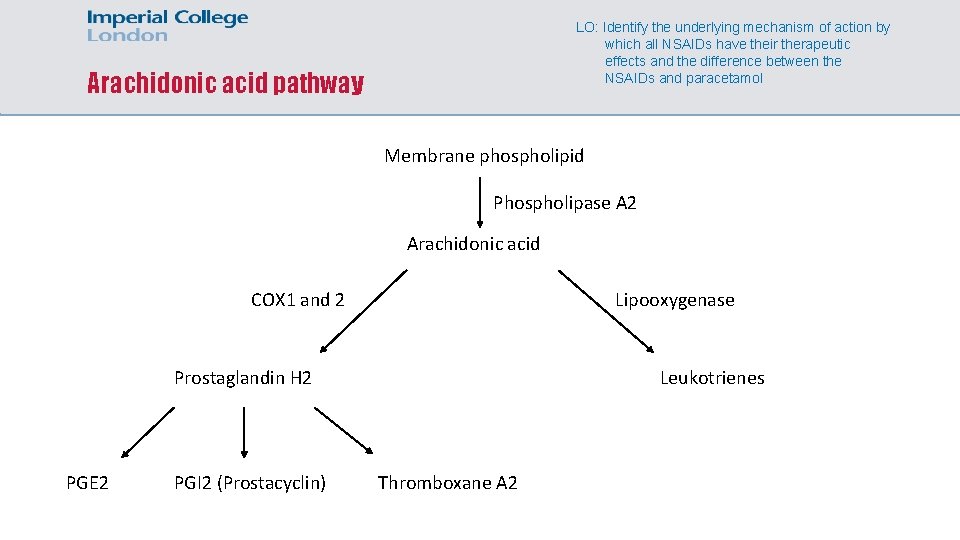
LO: Identify the underlying mechanism of action by which all NSAIDs have their therapeutic effects and the difference between the NSAIDs and paracetamol Arachidonic acid pathway Membrane phospholipid Phospholipase A 2 Arachidonic acid COX 1 and 2 Lipooxygenase Leukotrienes Prostaglandin H 2 PGE 2 PGI 2 (Prostacyclin) Thromboxane A 2

LO: Identify the underlying mechanism of action by which all NSAIDs have their therapeutic effects and the difference between the NSAIDs and paracetamol PGE 2 • PGE 2 o Lowers the pain threshold o Increases body temperature (fever!) o Causes inflammation o Activates T-helper cells involved in certain hypersensitive conditions

LO: Identify the underlying mechanism of action by which all NSAIDs have their therapeutic effects and the difference between the NSAIDs and paracetamol PGE 2 • PGE 2 o Is cytoprotective in the stomach! o Causes bronchodilation a long with other prostaglandins o Increases GFR
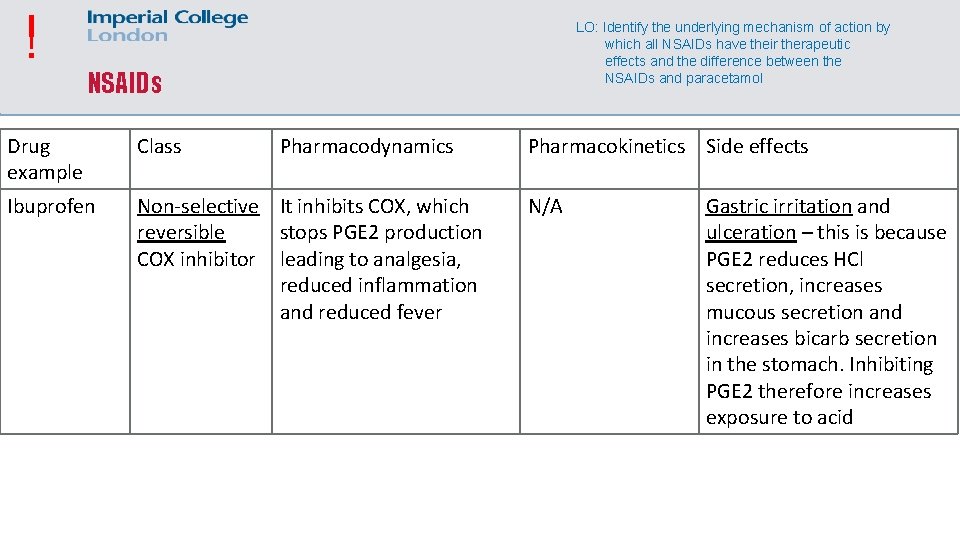
! LO: Identify the underlying mechanism of action by which all NSAIDs have their therapeutic effects and the difference between the NSAIDs and paracetamol NSAIDs Drug example Class Pharmacodynamics Ibuprofen Non-selective It inhibits COX, which reversible stops PGE 2 production COX inhibitor leading to analgesia, reduced inflammation and reduced fever Pharmacokinetics Side effects N/A Gastric irritation and ulceration – this is because PGE 2 reduces HCl secretion, increases mucous secretion and increases bicarb secretion in the stomach. Inhibiting PGE 2 therefore increases exposure to acid
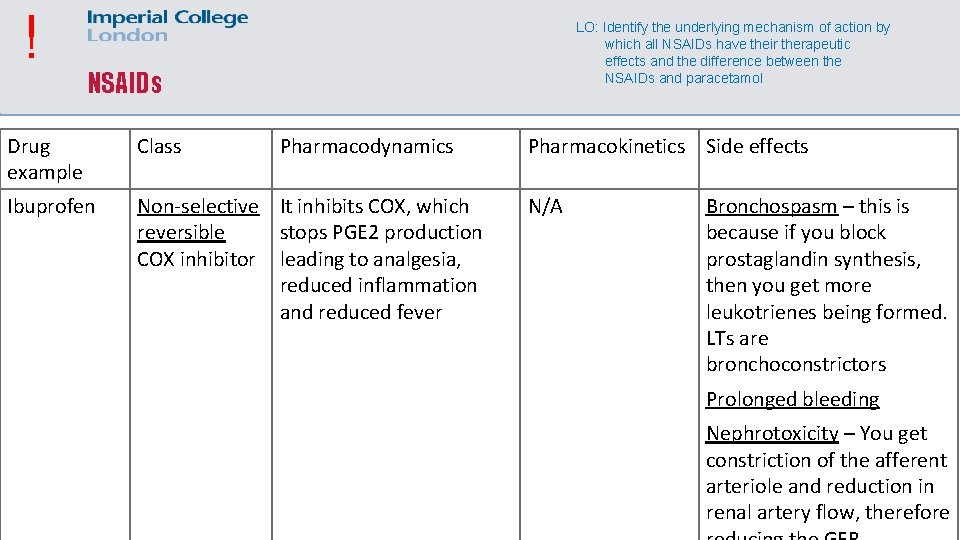
! LO: Identify the underlying mechanism of action by which all NSAIDs have their therapeutic effects and the difference between the NSAIDs and paracetamol NSAIDs Drug example Class Pharmacodynamics Ibuprofen Non-selective It inhibits COX, which reversible stops PGE 2 production COX inhibitor leading to analgesia, reduced inflammation and reduced fever Pharmacokinetics Side effects N/A Bronchospasm – this is because if you block prostaglandin synthesis, then you get more leukotrienes being formed. LTs are bronchoconstrictors Prolonged bleeding Nephrotoxicity – You get constriction of the afferent arteriole and reduction in renal artery flow, therefore
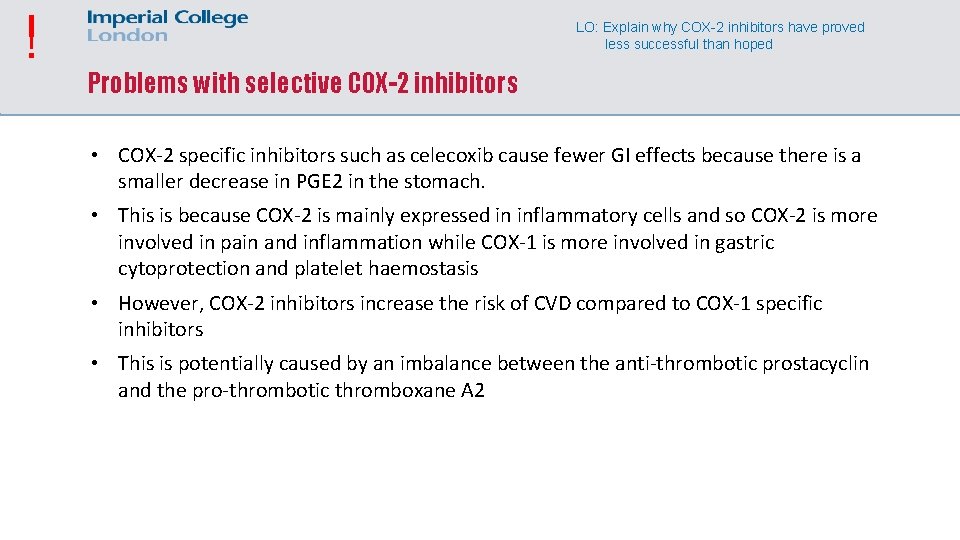
! LO: Explain why COX-2 inhibitors have proved less successful than hoped Problems with selective COX-2 inhibitors • COX-2 specific inhibitors such as celecoxib cause fewer GI effects because there is a smaller decrease in PGE 2 in the stomach. • This is because COX-2 is mainly expressed in inflammatory cells and so COX-2 is more involved in pain and inflammation while COX-1 is more involved in gastric cytoprotection and platelet haemostasis • However, COX-2 inhibitors increase the risk of CVD compared to COX-1 specific inhibitors • This is potentially caused by an imbalance between the anti-thrombotic prostacyclin and the pro-thrombotic thromboxane A 2
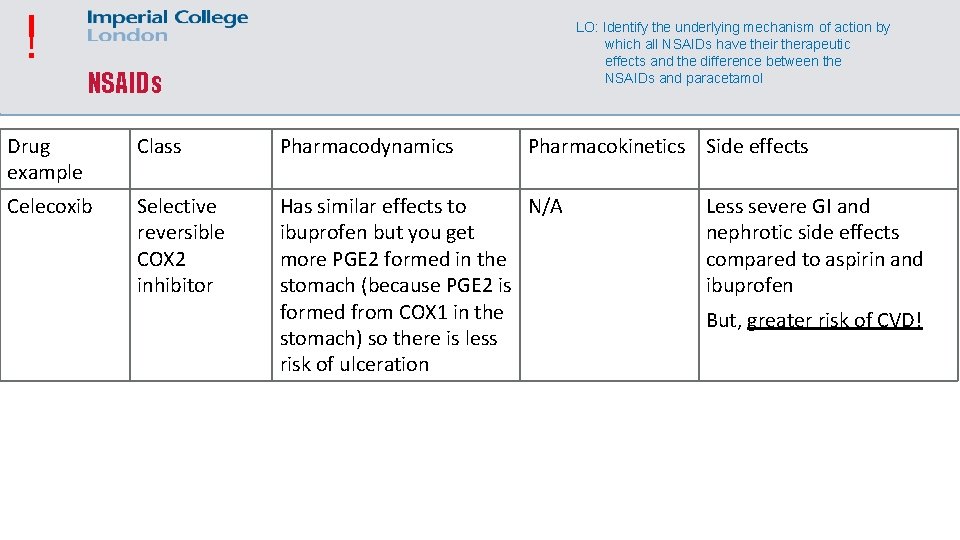
! LO: Identify the underlying mechanism of action by which all NSAIDs have their therapeutic effects and the difference between the NSAIDs and paracetamol NSAIDs Drug example Class Pharmacodynamics Pharmacokinetics Side effects Celecoxib Selective reversible COX 2 inhibitor Has similar effects to N/A ibuprofen but you get more PGE 2 formed in the stomach (because PGE 2 is formed from COX 1 in the stomach) so there is less risk of ulceration Less severe GI and nephrotic side effects compared to aspirin and ibuprofen But, greater risk of CVD!

! Paracetamol LO: Identify the underlying mechanism of action by which all NSAIDs have their therapeutic effects and the difference between the NSAIDs and paracetamol • Paracetamol doesn’t inhibit COX (unlike NSAIDs) • No one knows how paracetamol works, but it likely acts centrally rather than peripherally (unlike NSAIDs)

! Paracetamol LO: Identify the underlying mechanism of action by which all NSAIDs have their therapeutic effects and the difference between the NSAIDs and paracetamol • Paracetamol metabolism produces a toxic metabolite that is usually inactivated by glutathione. If this metabolite is produced in excess (e. g. in paracetamol overdose), then the metabolite starts binding to hepatic enzymes with –SH groups on them. This leads to liver failure • Acetylcysteine contains an –SH group, and so mops up the toxic metabolite during paracetamol overdose
! Why is aspirin the only NSAID used to manage thrombotic disease? • Aspirin irreversibly binds to COX 1 • Platelets lack a nucleus and so cannot resynthesise COX 1 • Irreversibly inhibiting COX 1 will permanently prevent TXA 2 production from the targeted platelets, but endothelial cells will be able to replenish their COX 1 and continue to produce prostacyclin, which is anti-thrombitic

A patient comes into A&E after overdosing on aspirin, why would you give IV sodium bicarbonate? A) To treat metabolic acidosis B) To increase urinary excretion of the drug C) To decrease the absorption of aspirin in the stomach D) To increase the stomach p. H to prevent ulceration E) To correct electrolyte imbalances

How do NSAIDs increase the risk of GI bleeding? A) Increased leukotriene formation B) Decreased secretion of cytoprotective bicarbonate C) Decreased HCl secretion D) Decreased platelet activation E) Upregulation of protein C and S

Atherosclerosis
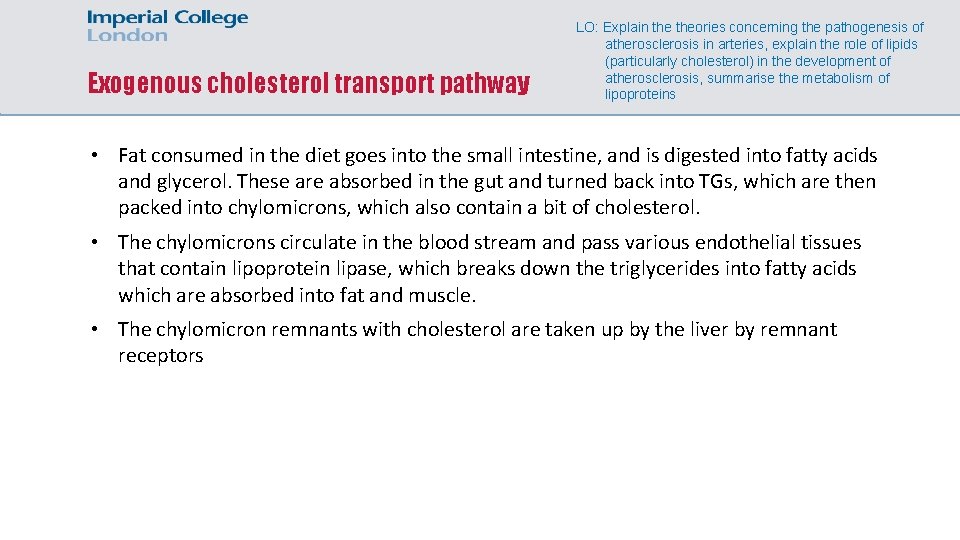
Exogenous cholesterol transport pathway LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins • Fat consumed in the diet goes into the small intestine, and is digested into fatty acids and glycerol. These are absorbed in the gut and turned back into TGs, which are then packed into chylomicrons, which also contain a bit of cholesterol. • The chylomicrons circulate in the blood stream and pass various endothelial tissues that contain lipoprotein lipase, which breaks down the triglycerides into fatty acids which are absorbed into fat and muscle. • The chylomicron remnants with cholesterol are taken up by the liver by remnant receptors
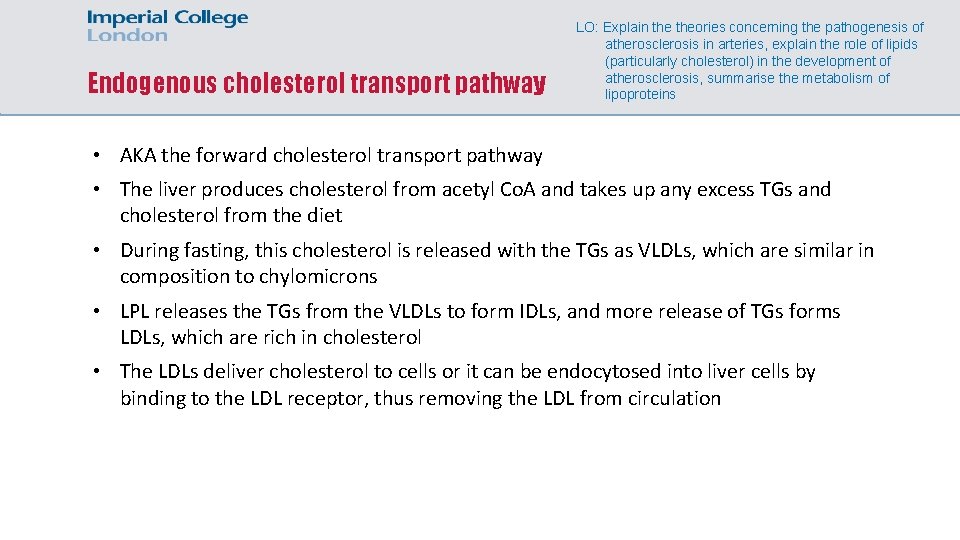
Endogenous cholesterol transport pathway LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins • AKA the forward cholesterol transport pathway • The liver produces cholesterol from acetyl Co. A and takes up any excess TGs and cholesterol from the diet • During fasting, this cholesterol is released with the TGs as VLDLs, which are similar in composition to chylomicrons • LPL releases the TGs from the VLDLs to form IDLs, and more release of TGs forms LDLs, which are rich in cholesterol • The LDLs deliver cholesterol to cells or it can be endocytosed into liver cells by binding to the LDL receptor, thus removing the LDL from circulation

Cholesterol ester transfer protein LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins • An enzyme called cholesterol ester transfer protein (CETP) is involved in forming small dense LDLs • CETP transfers TGs from VLDLs/chylomicrons/HDLs to LDLs • Lipases releases these TGs from the LDL, leaving behind a small and dense LDL particle
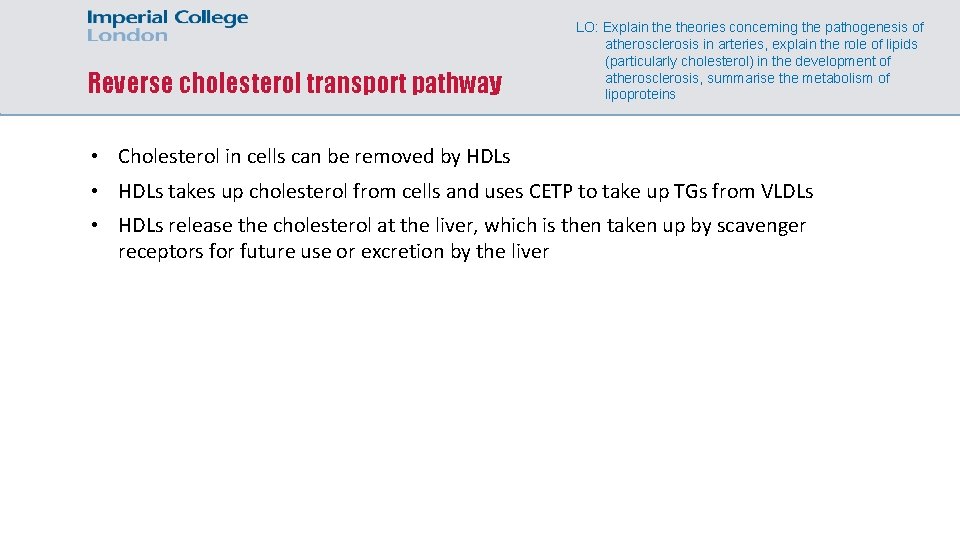
Reverse cholesterol transport pathway LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins • Cholesterol in cells can be removed by HDLs • HDLs takes up cholesterol from cells and uses CETP to take up TGs from VLDLs • HDLs release the cholesterol at the liver, which is then taken up by scavenger receptors for future use or excretion by the liver
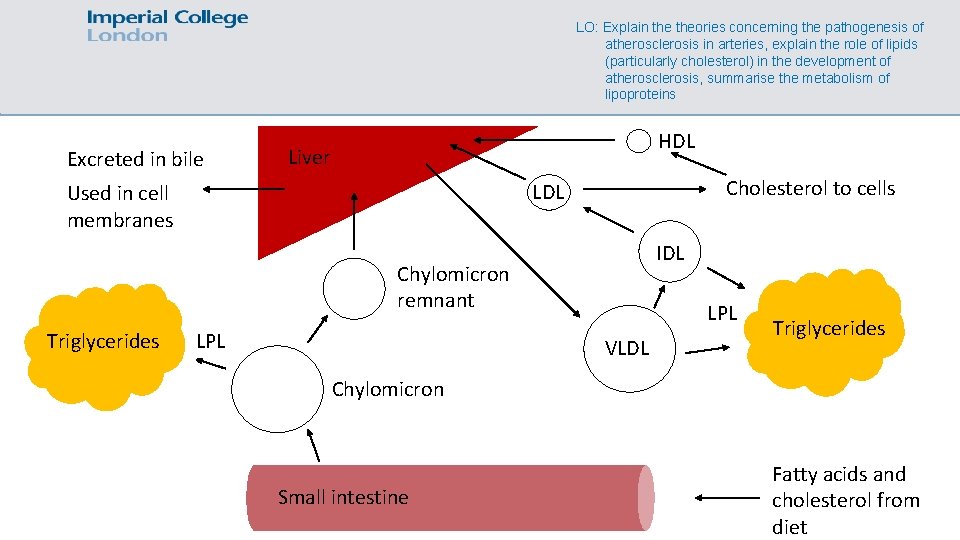
LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins Excreted in bile HDL Liver Cholesterol to cells LDL Used in cell membranes IDL Chylomicron remnant Triglycerides LPL VLDL Triglycerides Chylomicron Small intestine Fatty acids and cholesterol from diet

Atherosclerosis formation LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins • Excess LDLs and small dense LDLs can get into the subendothelial layer of an artery (tunica intima), particularly in places that are prone to atheroma formation • These prone areas are highly oxidative, so the LDLs are oxidised by free radicals. • Endothelial cells detect this and recruit monocytes, which move into the intima • The monocytes become macrophages in tissue and use a scavenger receptor to phagocytose the oxidised LDLs to form foam cells. This stimulates chronic inflammation leading to a vicious cycle of lipoprotein oxidisation, macrophage recruitment and phagocytosis.

Atherosclerosis formation LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins • Macrophages stimulate proliferation of smooth muscle cells and increased collagen synthesis as part of their wound healing role • The proliferation of these smooth muscle cells and extra collagen forms a fibrous cap around the mass of foam cells (“fatty streak”) • This mass can rupture through the cap and the endothelium, leading to thrombosis
Atherosclerosis formation LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins • Chylomicron remnants, IDLs, LDLs and small dense LDLs are highly atherogenic • Saturated fats increase the amount of these lipoproteins, hence why there is increased risk of cardiovascular disease
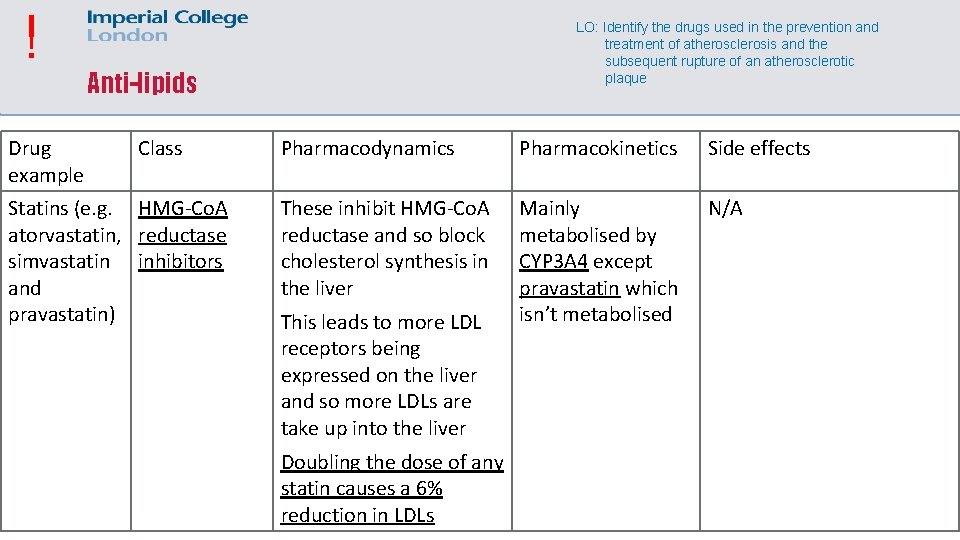
! Drug example LO: Identify the drugs used in the prevention and treatment of atherosclerosis and the subsequent rupture of an atherosclerotic plaque Anti-lipids Class Statins (e. g. HMG-Co. A atorvastatin, reductase simvastatin inhibitors and pravastatin) Pharmacodynamics Pharmacokinetics Side effects These inhibit HMG-Co. A reductase and so block cholesterol synthesis in the liver Mainly metabolised by CYP 3 A 4 except pravastatin which isn’t metabolised N/A This leads to more LDL receptors being expressed on the liver and so more LDLs are take up into the liver Doubling the dose of any statin causes a 6% reduction in LDLs
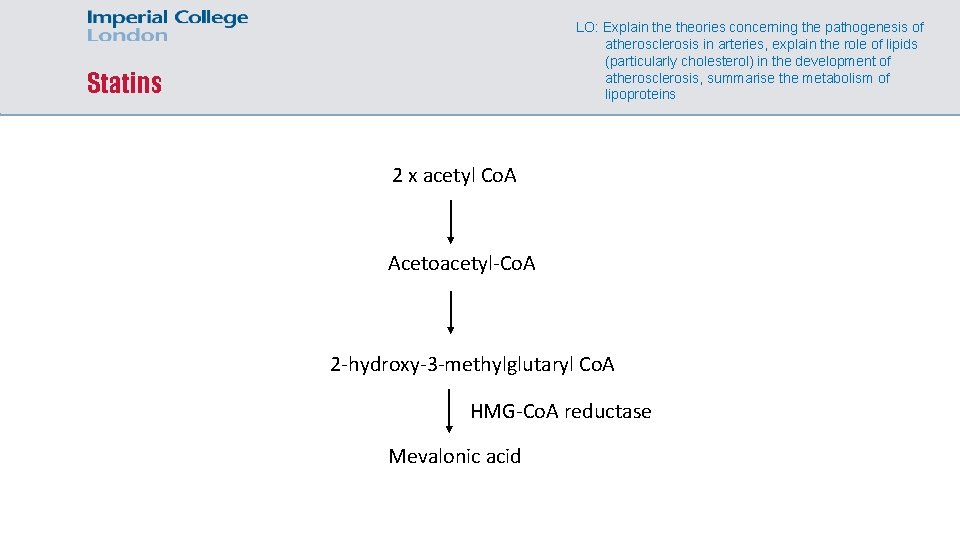
LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins Statins 2 x acetyl Co. A Acetoacetyl-Co. A 2 -hydroxy-3 -methylglutaryl Co. A HMG-Co. A reductase Mevalonic acid
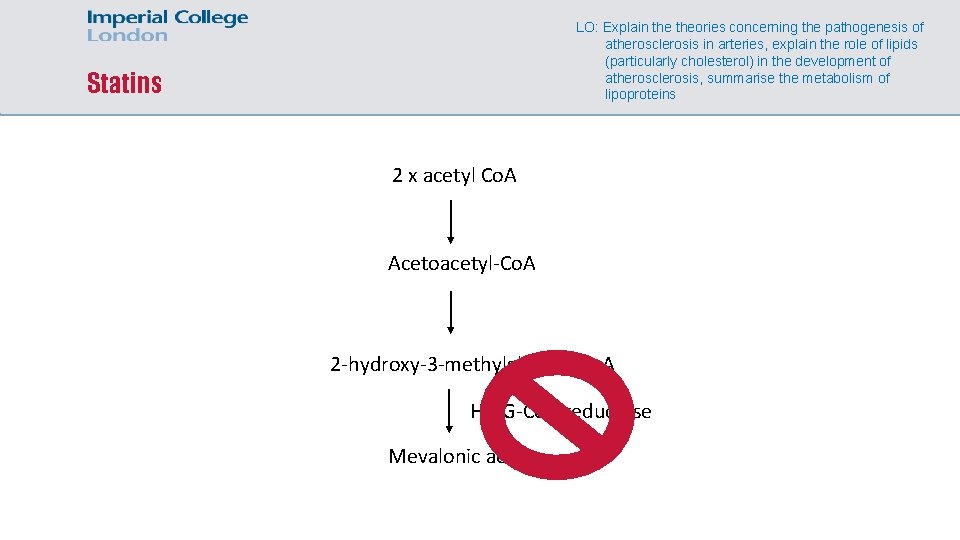
LO: Explain theories concerning the pathogenesis of atherosclerosis in arteries, explain the role of lipids (particularly cholesterol) in the development of atherosclerosis, summarise the metabolism of lipoproteins Statins 2 x acetyl Co. A Acetoacetyl-Co. A 2 -hydroxy-3 -methylglutaryl Co. A HMG-Co. A reductase Mevalonic acid
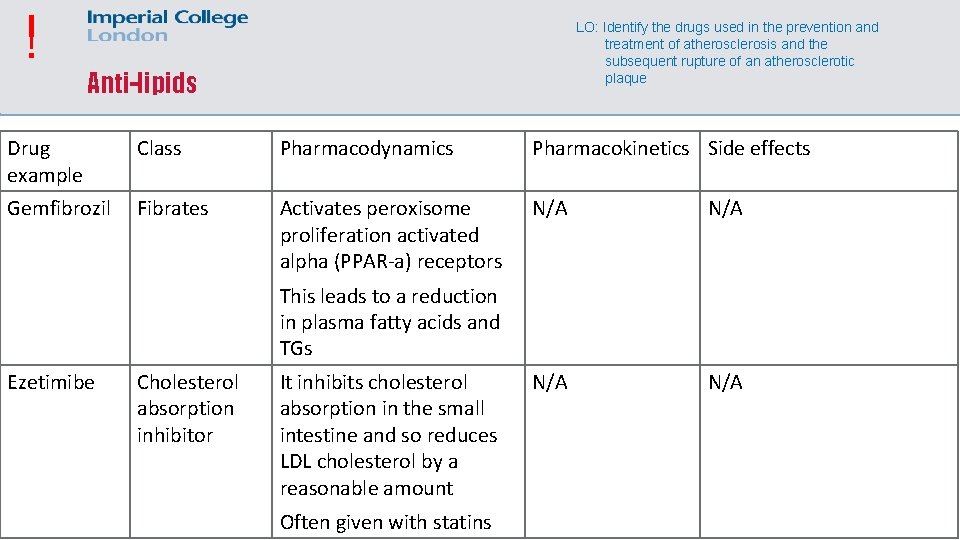
! LO: Identify the drugs used in the prevention and treatment of atherosclerosis and the subsequent rupture of an atherosclerotic plaque Anti-lipids Drug example Class Pharmacodynamics Pharmacokinetics Side effects Gemfibrozil Fibrates Activates peroxisome proliferation activated alpha (PPAR-a) receptors N/A N/A This leads to a reduction in plasma fatty acids and TGs Ezetimibe Cholesterol absorption inhibitor It inhibits cholesterol absorption in the small intestine and so reduces LDL cholesterol by a reasonable amount Often given with statins
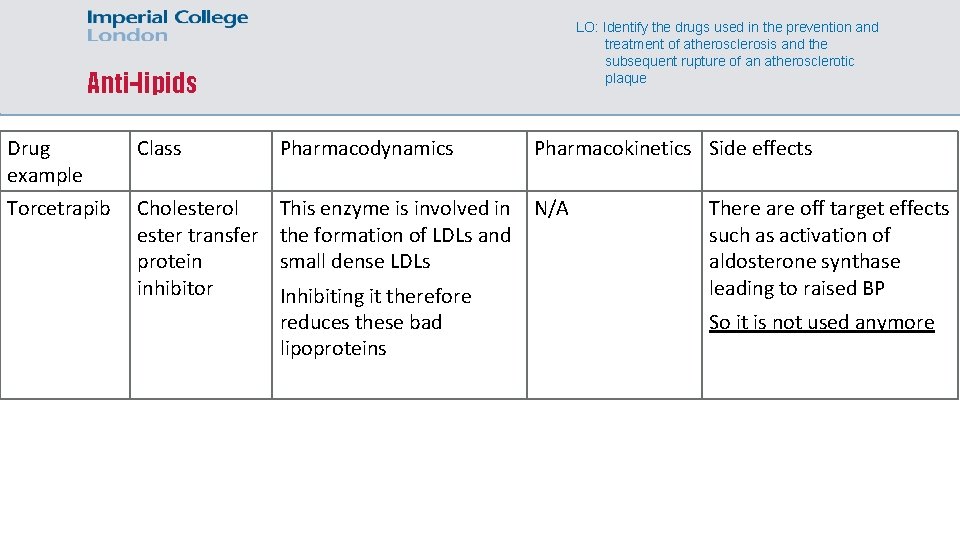
LO: Identify the drugs used in the prevention and treatment of atherosclerosis and the subsequent rupture of an atherosclerotic plaque Anti-lipids Drug example Class Pharmacodynamics Pharmacokinetics Side effects Torcetrapib Cholesterol ester transfer protein inhibitor This enzyme is involved in the formation of LDLs and small dense LDLs N/A Inhibiting it therefore reduces these bad lipoproteins There are off target effects such as activation of aldosterone synthase leading to raised BP So it is not used anymore

Use of statins causes a build up of what in the cholesterol synthesis pathway? A) Mevalonic acid B) Squalene C) Farnesyl pyrophosphate D) HMG-Co. A E) Isopentenyl pyrophosphate

What is the mechanism of action of fibrates? A) Activate CETP B) Inhibit PPAR-alpha C) Inhibit cholesterol absorption in the intestine D) Activate PPAR-alpha E) Inhibit CETP
Which of these is the least atherogenic? A) LDL B) Small dense LDL C) VLDL D) IDL E) Chylomicron remnant
Feedback pls: bit. ly/muslimmedics Brainscape link: https: //www. brainscape. com/profiles/2683619 My email: ailbe. smith 15@imperial. ac. uk
- Slides: 54