Year 10 Paper 1 Combined Chemistry Foundation Revision

Year 10 (Paper 1) Combined Chemistry Foundation Revision Name…………………
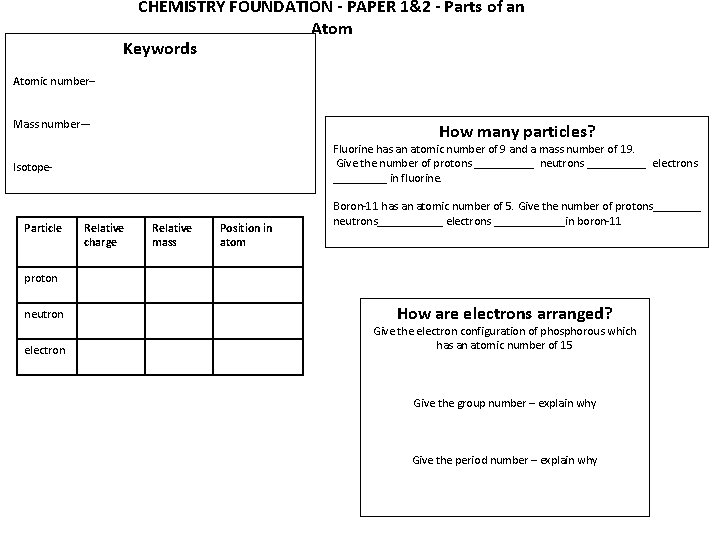
CHEMISTRY FOUNDATION - PAPER 1&2 - Parts of an Atom Keywords Atomic number– Mass number— How many particles? Fluorine has an atomic number of 9 and a mass number of 19. Give the number of protons _____ neutrons _____ electrons _____ in fluorine. Isotope- Particle Relative charge Relative mass Position in atom Boron-11 has an atomic number of 5. Give the number of protons____ neutrons______ electrons ______in boron-11 proton neutron electron How are electrons arranged? Give the electron configuration of phosphorous which has an atomic number of 15 Give the group number – explain why Give the period number – explain why
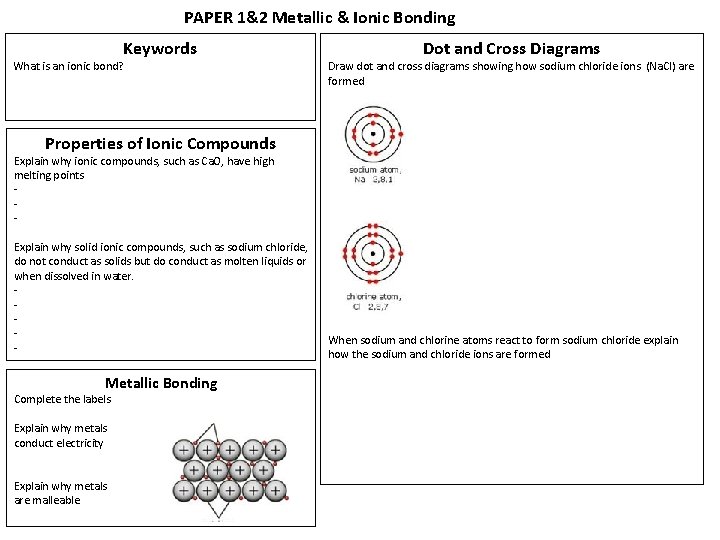
PAPER 1&2 Metallic & Ionic Bonding Keywords What is an ionic bond? Dot and Cross Diagrams Draw dot and cross diagrams showing how sodium chloride ions (Na. Cl) are formed Properties of Ionic Compounds Explain why ionic compounds, such as Ca. O, have high melting points - Explain why solid ionic compounds, such as sodium chloride, do not conduct as solids but do conduct as molten liquids or when dissolved in water. - Metallic Bonding Complete the labels Explain why metals conduct electricity Explain why metals are malleable When sodium and chlorine atoms react to form sodium chloride explain how the sodium and chloride ions are formed
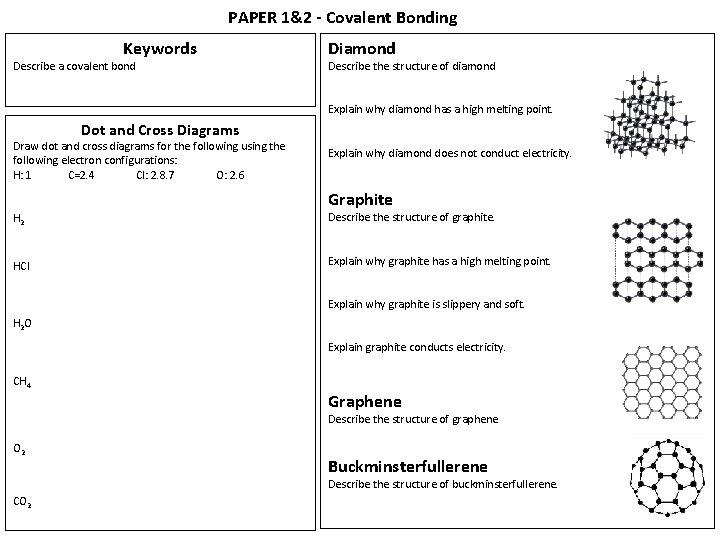
PAPER 1&2 - Covalent Bonding Keywords Describe a covalent bond Diamond Describe the structure of diamond Explain why diamond has a high melting point. Dot and Cross Diagrams Draw dot and cross diagrams for the following using the following electron configurations: H: 1 C=2. 4 Cl: 2. 8. 7 O: 2. 6 Explain why diamond does not conduct electricity. Graphite H 2 Describe the structure of graphite. HCl Explain why graphite has a high melting point. Explain why graphite is slippery and soft. H 2 O Explain graphite conducts electricity. CH 4 Graphene Describe the structure of graphene O 2 Buckminsterfullerene Describe the structure of buckminsterfullerene. CO 2

PAPER 1&2 - Calculation Questions Give the empirical formula of C 5 H 10 Calculate the Relative Formula Mass of Ca(NO 3)2. (Relative atomic mass Ca= 40, N=14, O=16 If 20 g of sodium chloride dissolves in 100 cm 3 of water calculate the concentration of the sodium chloride solution in gdm-3 In a chromatogram if the solvent moved 15 cm and the solute moved 10 cm calculate the Rf value If 5. 3 g of magnesium reacted with 15. 7 g of chlorine calculate the empirical formula (Relative atomic mass of Mg=24, Cl=35. 5) Ca. CO 3 + HCl Ca. Cl 2 + CO 2 + H 2 O If 2 g of calcium carbonate reacts in the above equation calculate the mass of calcium chloride produced. (Relative atomic mass Ca: 40, C: 12, O 16, H=1, Cl=35. 5)
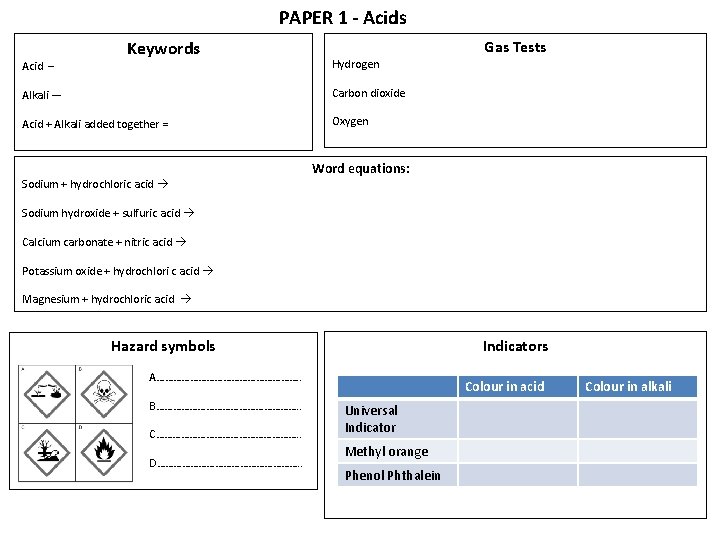
PAPER 1 - Acids Acid – Keywords Hydrogen Alkali — Carbon dioxide Acid + Alkali added together = Oxygen Sodium + hydrochloric acid Gas Tests Word equations: Sodium hydroxide + sulfuric acid Calcium carbonate + nitric acid Potassium oxide + hydrochlori c acid Magnesium + hydrochloric acid Indicators Hazard symbols A………………………. . B………………………. . C………………………. . D………………………. . Colour in acid Universal Indicator Methyl orange Phenol Phthalein Colour in alkali
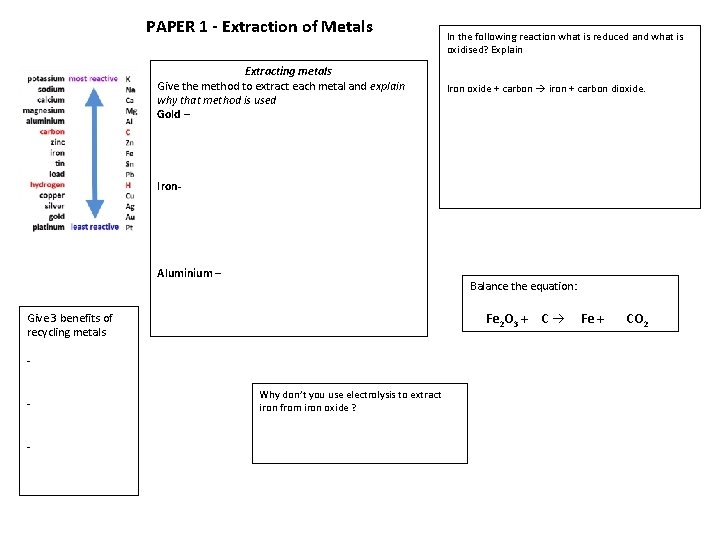
PAPER 1 - Extraction of Metals Extracting metals Give the method to extract each metal and explain why that method is used Gold – In the following reaction what is reduced and what is oxidised? Explain Iron oxide + carbon iron + carbon dioxide. Iron- Aluminium – Balance the equation: Fe 2 O 3 + C Give 3 benefits of recycling metals - Why don’t you use electrolysis to extract iron from iron oxide ? Fe + CO 2
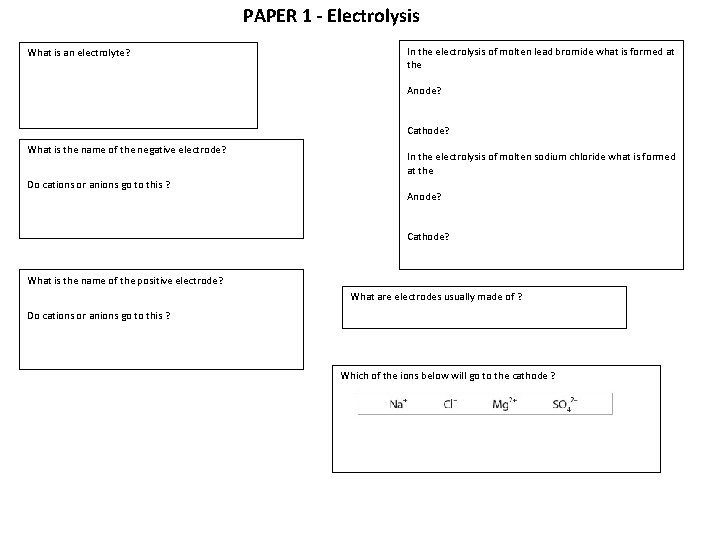
PAPER 1 - Electrolysis What is an electrolyte? In the electrolysis of molten lead bromide what is formed at the Anode? Cathode? What is the name of the negative electrode? Do cations or anions go to this ? In the electrolysis of molten sodium chloride what is formed at the Anode? Cathode? What is the name of the positive electrode? What are electrodes usually made of ? Do cations or anions go to this ? Which of the ions below will go to the cathode ?
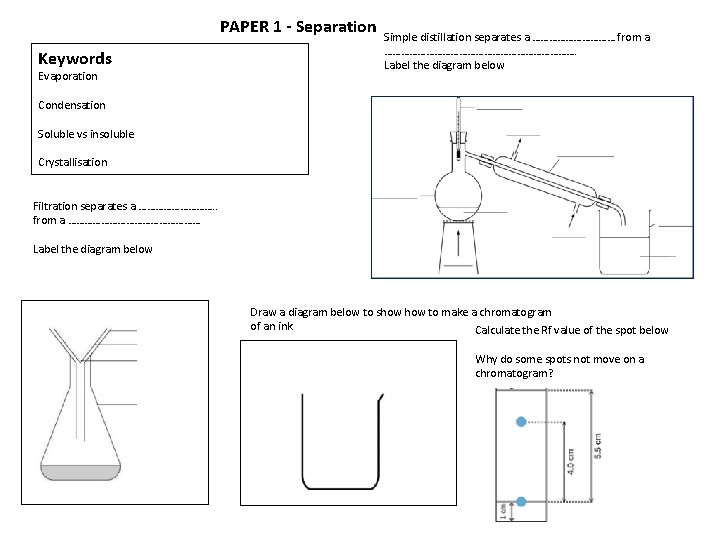
PAPER 1 - Separation Keywords Evaporation Simple distillation separates a …………… from a ………………………………. Label the diagram below Condensation Soluble vs insoluble Crystallisation Filtration separates a ……………. . from a …………………… Label the diagram below Draw a diagram below to show to make a chromatogram of an ink. Calculate the Rf value of the spot below Why do some spots not move on a chromatogram?
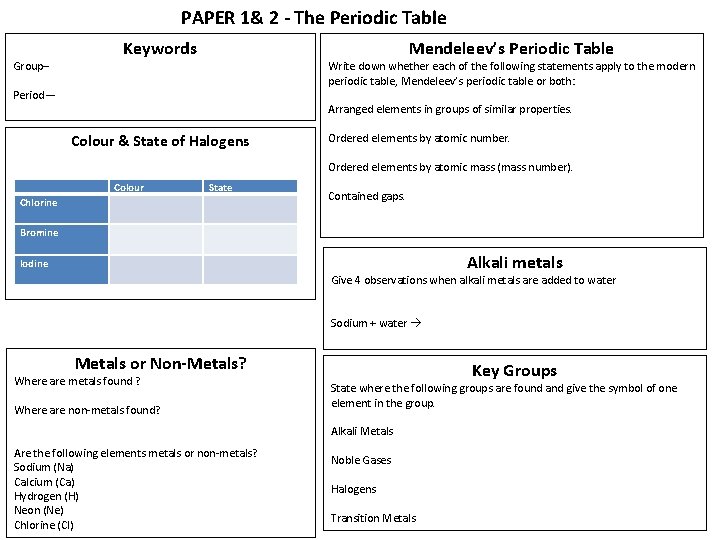
PAPER 1& 2 - The Periodic Table Group– Mendeleev’s Periodic Table Keywords Write down whether each of the following statements apply to the modern periodic table, Mendeleev’s periodic table or both: Period— Arranged elements in groups of similar properties. Colour & State of Halogens Ordered elements by atomic number. Ordered elements by atomic mass (mass number). Colour State Chlorine Contained gaps. Bromine Alkali metals Iodine Give 4 observations when alkali metals are added to water Sodium + water Metals or Non-Metals? Where are metals found ? Where are non-metals found? Key Groups State where the following groups are found and give the symbol of one element in the group. Alkali Metals Are the following elements metals or non-metals? Sodium (Na) Calcium (Ca) Hydrogen (H) Neon (Ne) Chlorine (Cl) Noble Gases Halogens Transition Metals
- Slides: 10