Xray Scattering Peak intensities Peak widths Second Annual







- Slides: 54

X-ray Scattering Peak intensities Peak widths Second Annual SSRL Workshop on Synchrotron X-ray Scattering Techniques in Materials and Environmental Sciences: Theory and Application Tuesday, May 15 - Thursday, May 17, 2007

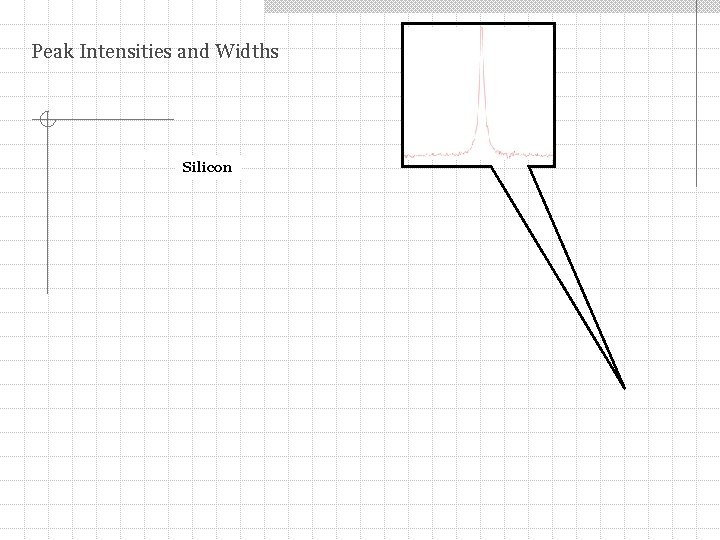
Peak Intensities and Widths Silicon

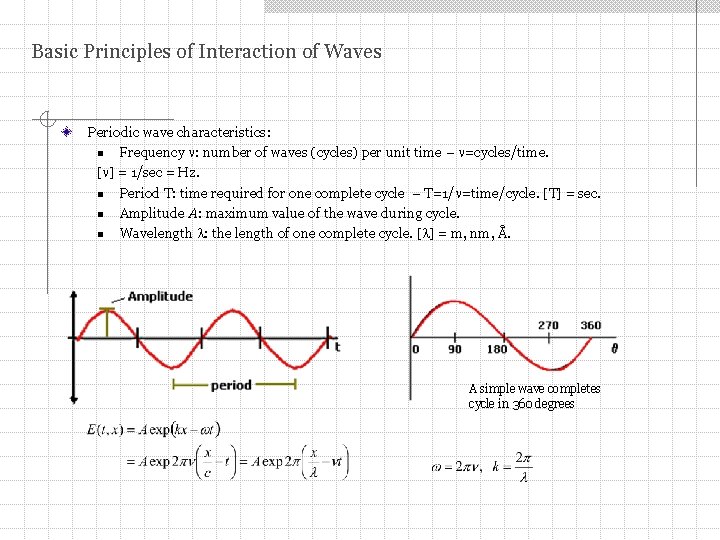
Basic Principles of Interaction of Waves Periodic wave characteristics: n Frequency : number of waves (cycles) per unit time – =cycles/time. [ ] = 1/sec = Hz. n Period T: time required for one complete cycle – T=1/ =time/cycle. [T] = sec. n Amplitude A: maximum value of the wave during cycle. n Wavelength : the length of one complete cycle. [ ] = m, nm, Å. A simple wave completes cycle in 360 degrees

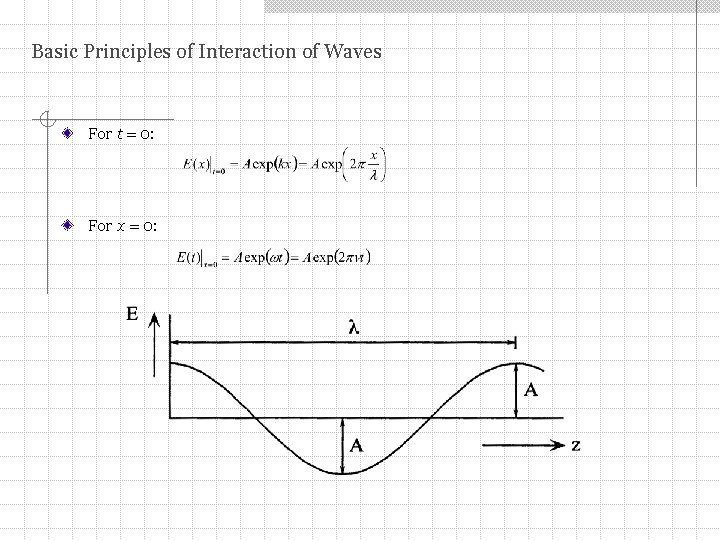
Basic Principles of Interaction of Waves For t = 0: For x = 0:

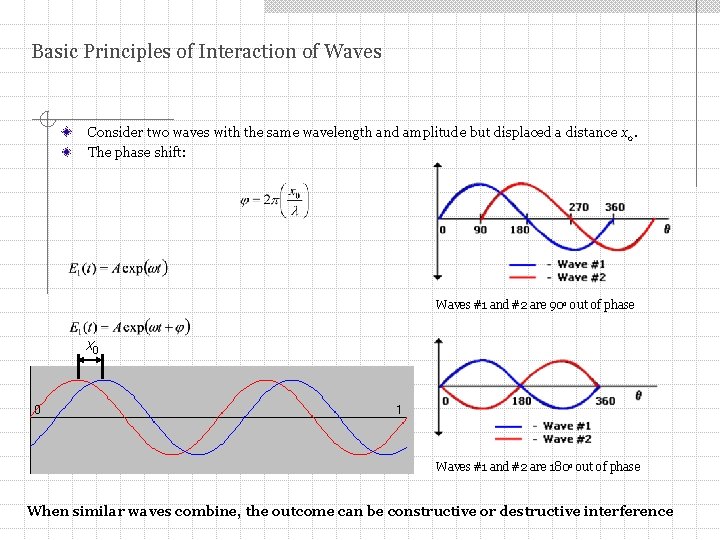
Basic Principles of Interaction of Waves Consider two waves with the same wavelength and amplitude but displaced a distance x 0. The phase shift: Waves #1 and #2 are 90 o out of phase x 0 Waves #1 and #2 are 180 o out of phase When similar waves combine, the outcome can be constructive or destructive interference

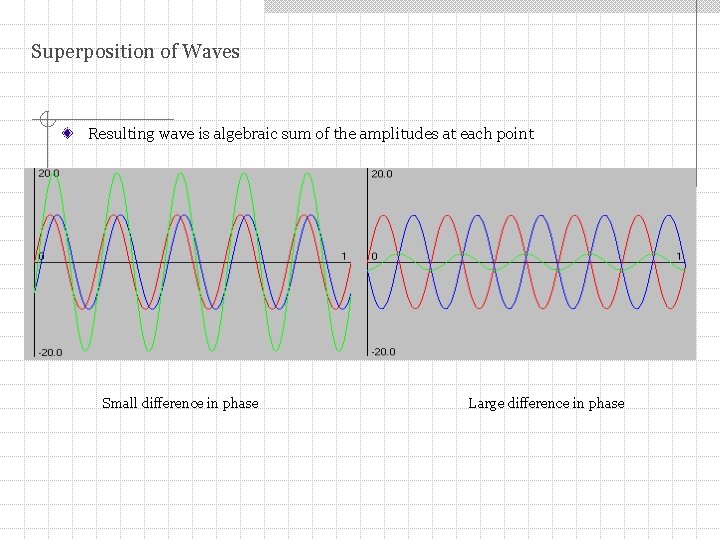
Superposition of Waves Resulting wave is algebraic sum of the amplitudes at each point Small difference in phase Large difference in phase

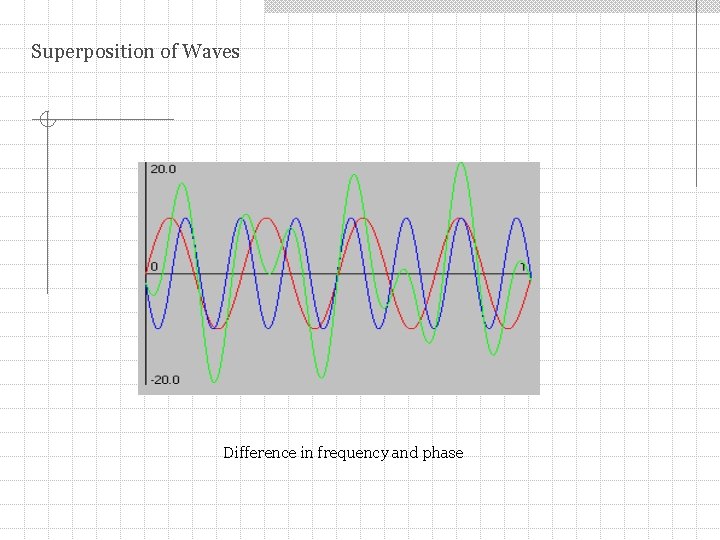
Superposition of Waves Difference in frequency and phase

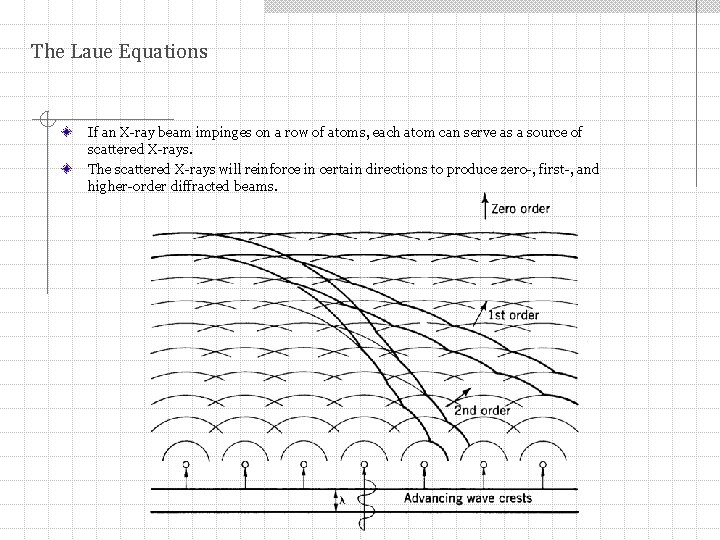
The Laue Equations If an X-ray beam impinges on a row of atoms, each atom can serve as a source of scattered X-rays. The scattered X-rays will reinforce in certain directions to produce zero-, first-, and higher-order diffracted beams.

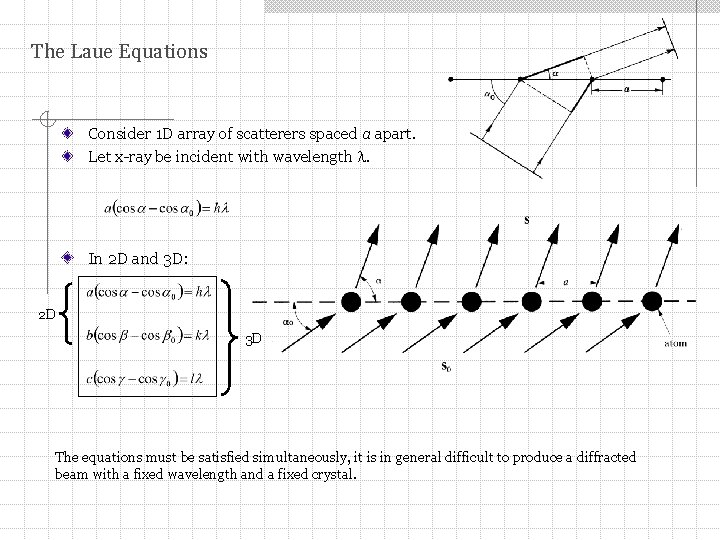
The Laue Equations Consider 1 D array of scatterers spaced a apart. Let x-ray be incident with wavelength . In 2 D and 3 D: 2 D 3 D The equations must be satisfied simultaneously, it is in general difficult to produce a diffracted beam with a fixed wavelength and a fixed crystal.

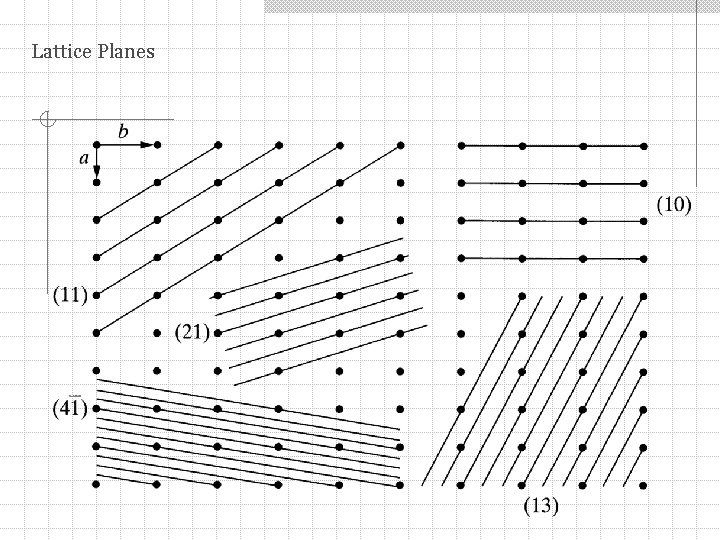
Lattice Planes

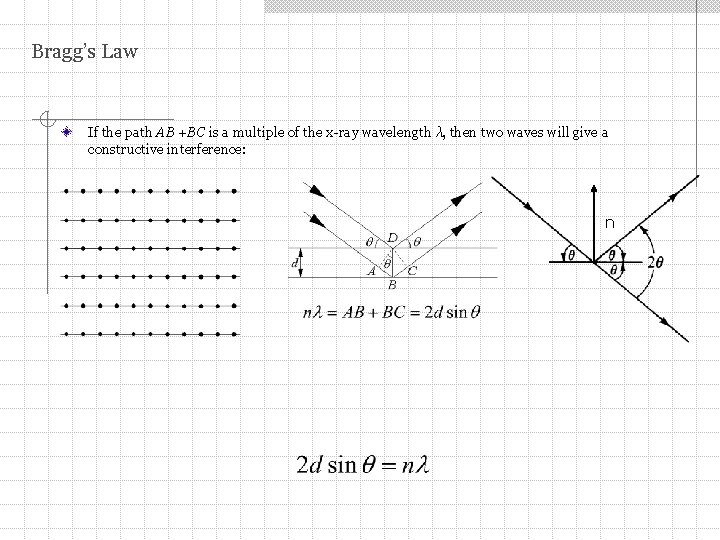
Bragg’s Law If the path AB +BC is a multiple of the x-ray wavelength λ, then two waves will give a constructive interference: n

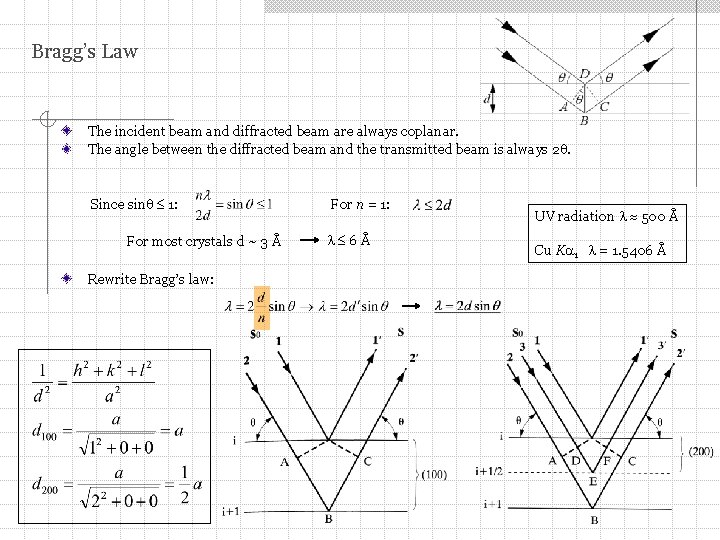
Bragg’s Law The incident beam and diffracted beam are always coplanar. The angle between the diffracted beam and the transmitted beam is always 2. Since sin 1: For most crystals d ~ 3 Å Rewrite Bragg’s law: For n = 1: 6Å UV radiation 500 Å Cu K 1 = 1. 5406 Å

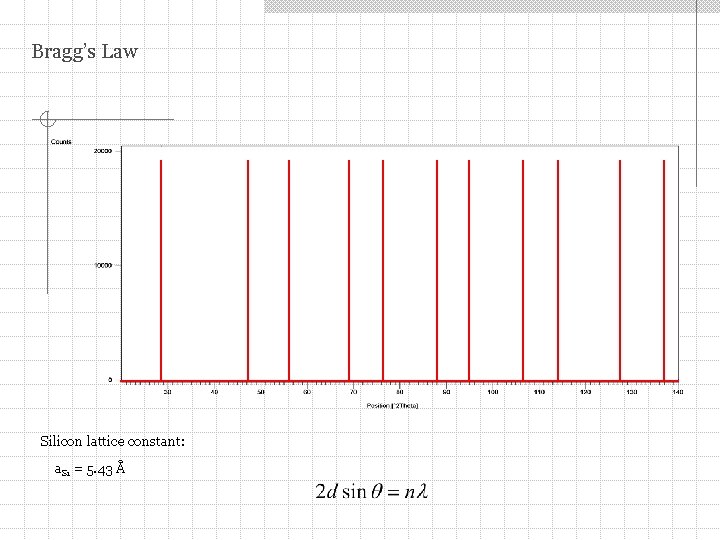
Bragg’s Law Silicon lattice constant: a. Si = 5. 43 Å

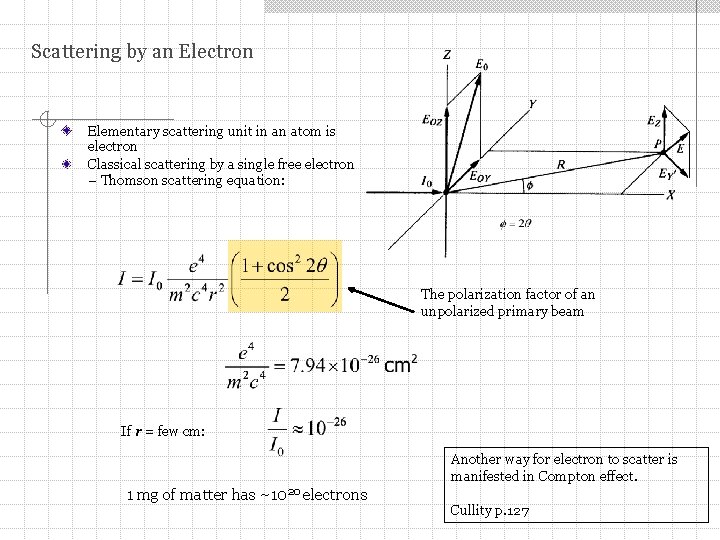
Scattering by an Electron Elementary scattering unit in an atom is electron Classical scattering by a single free electron – Thomson scattering equation: The polarization factor of an unpolarized primary beam If r = few cm: Another way for electron to scatter is manifested in Compton effect. 1 mg of matter has ~1020 electrons Cullity p. 127

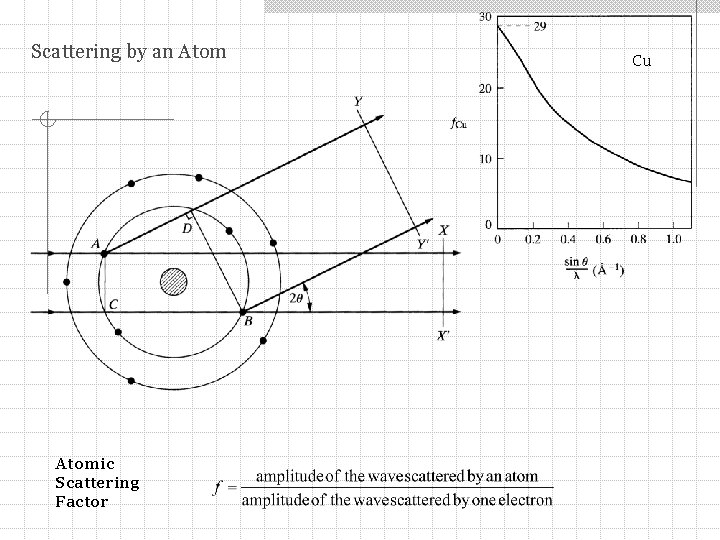
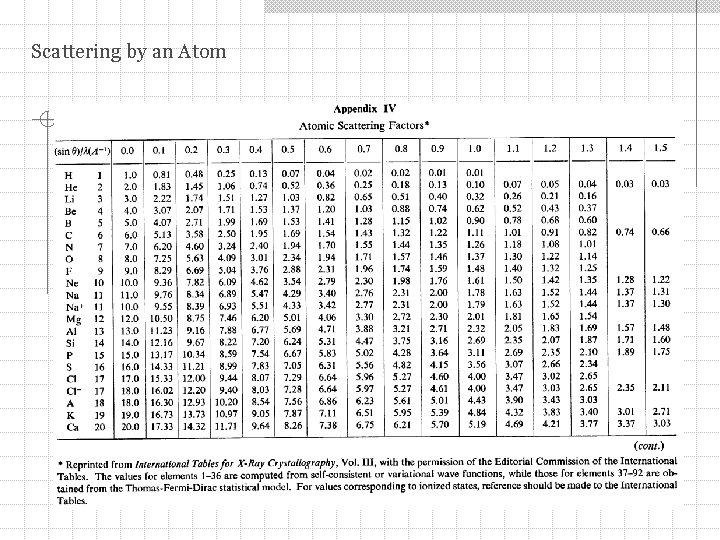
Scattering by an Atomic Scattering Factor Cu

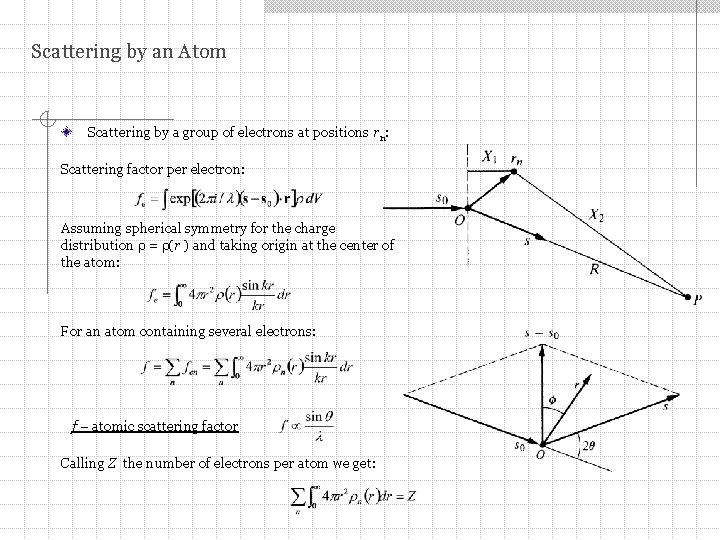
Scattering by an Atom Scattering by a group of electrons at positions rn: Scattering factor per electron: Assuming spherical symmetry for the charge distribution = (r ) and taking origin at the center of the atom: For an atom containing several electrons: f – atomic scattering factor Calling Z the number of electrons per atom we get:

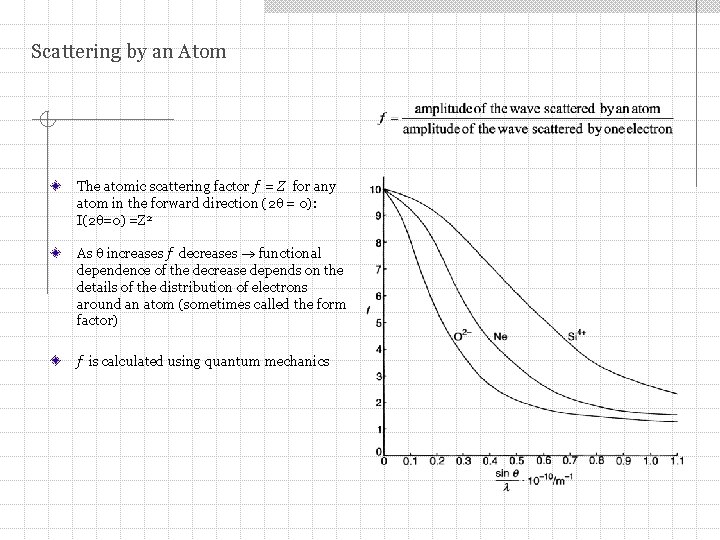
Scattering by an Atom The atomic scattering factor f = Z for any atom in the forward direction (2 = 0): I(2 =0) =Z 2 As increases f decreases functional dependence of the decrease depends on the details of the distribution of electrons around an atom (sometimes called the form factor) f is calculated using quantum mechanics

Scattering by an Atom

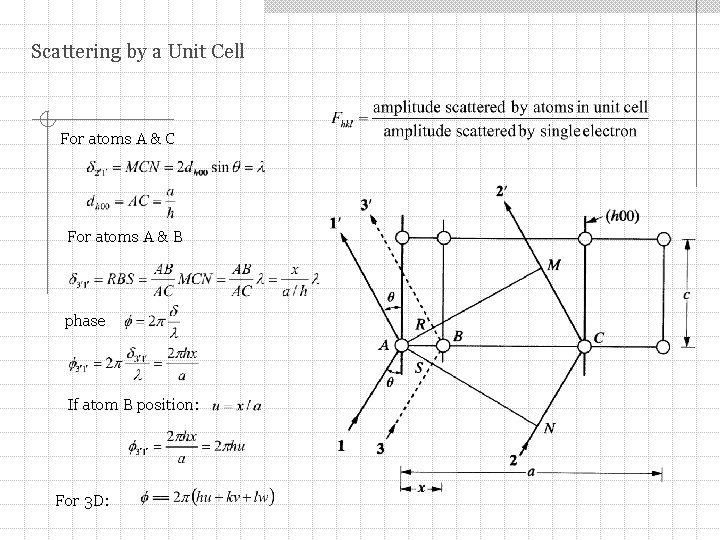
Scattering by a Unit Cell For atoms A & C For atoms A & B phase If atom B position: For 3 D:

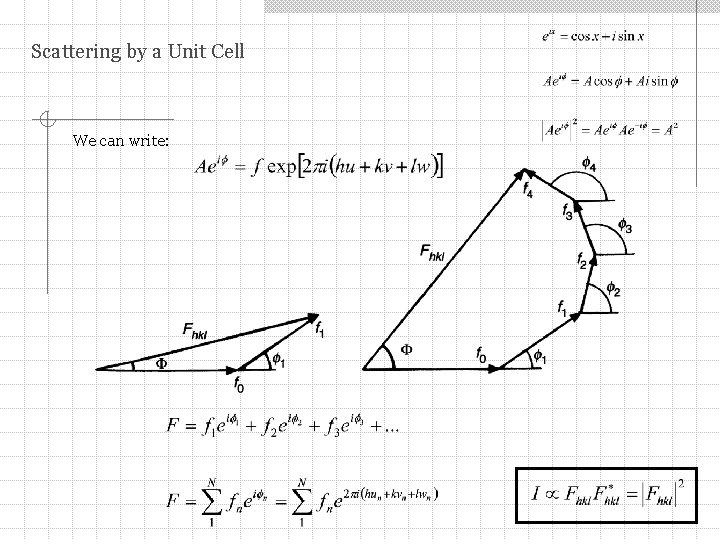
Scattering by a Unit Cell We can write:

Scattering by a Unit Cell Useful expressions Hint: don’t try to use the trigonometric form – using exponentials is much easier

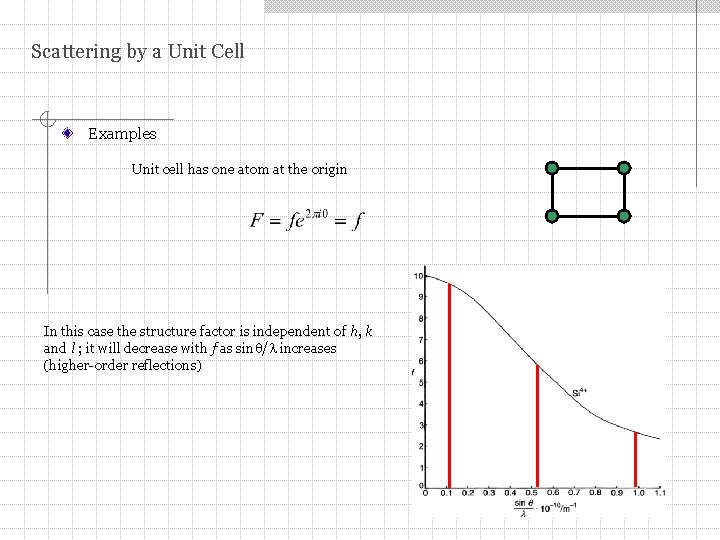
Scattering by a Unit Cell Examples Unit cell has one atom at the origin In this case the structure factor is independent of h, k and l ; it will decrease with f as sin / increases (higher-order reflections)

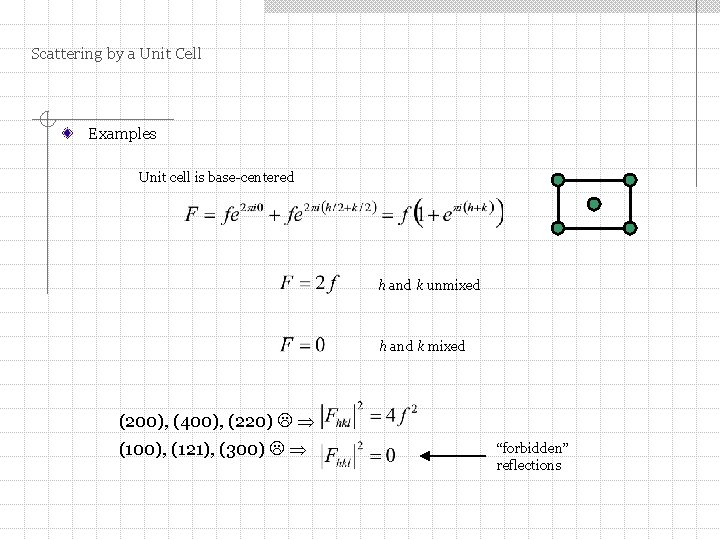
Scattering by a Unit Cell Examples Unit cell is base-centered h and k unmixed h and k mixed (200), (400), (220) (100), (121), (300) “forbidden” reflections

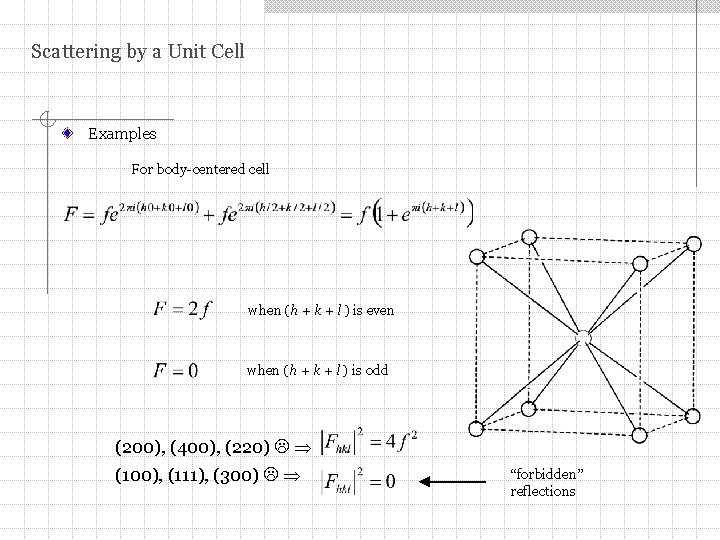
Scattering by a Unit Cell Examples For body-centered cell when (h + k + l ) is even when (h + k + l ) is odd (200), (400), (220) (100), (111), (300) “forbidden” reflections

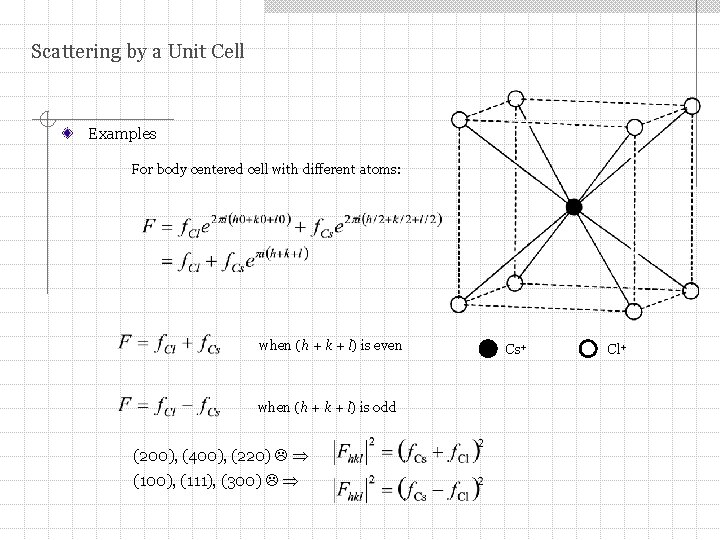
Scattering by a Unit Cell Examples For body centered cell with different atoms: when (h + k + l) is even when (h + k + l) is odd (200), (400), (220) (100), (111), (300) Cs+ Cl+

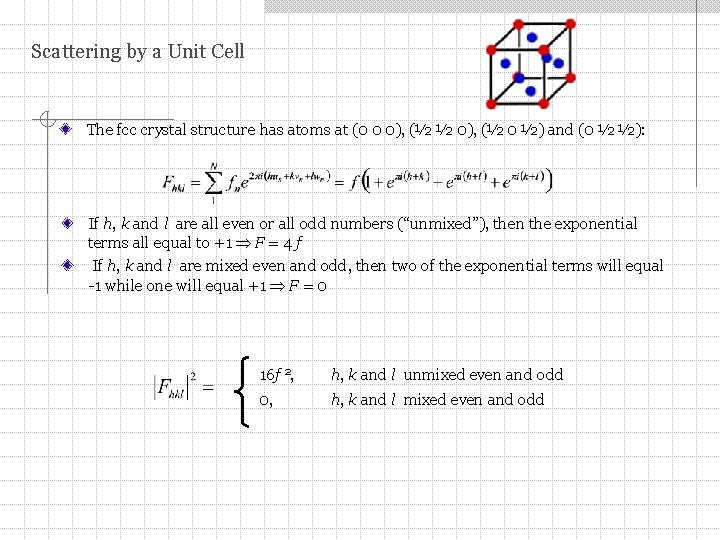
Scattering by a Unit Cell The fcc crystal structure has atoms at (0 0 0), (½ ½ 0), (½ 0 ½) and (0 ½ ½): If h, k and l are all even or all odd numbers (“unmixed”), then the exponential terms all equal to +1 F = 4 f If h, k and l are mixed even and odd, then two of the exponential terms will equal -1 while one will equal +1 F = 0 16 f 2, h, k and l unmixed even and odd 0, h, k and l mixed even and odd

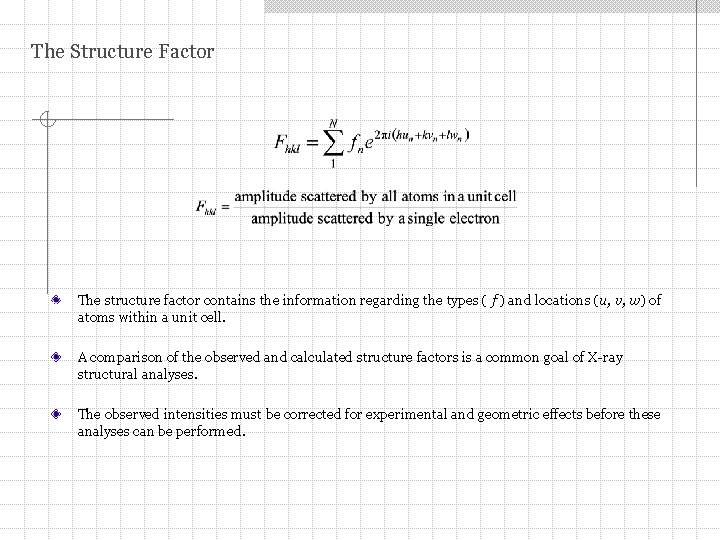
The Structure Factor The structure factor contains the information regarding the types ( f ) and locations (u, v, w) of atoms within a unit cell. A comparison of the observed and calculated structure factors is a common goal of X-ray structural analyses. The observed intensities must be corrected for experimental and geometric effects before these analyses can be performed.

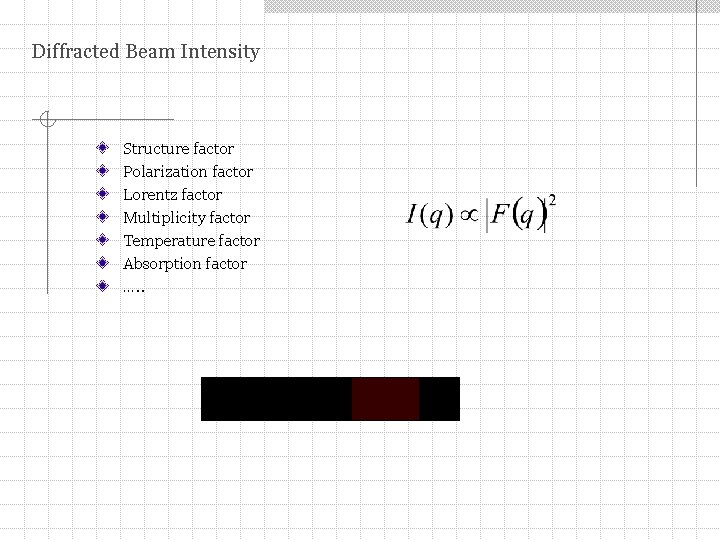
Diffracted Beam Intensity Structure factor Polarization factor Lorentz factor Multiplicity factor Temperature factor Absorption factor …. .

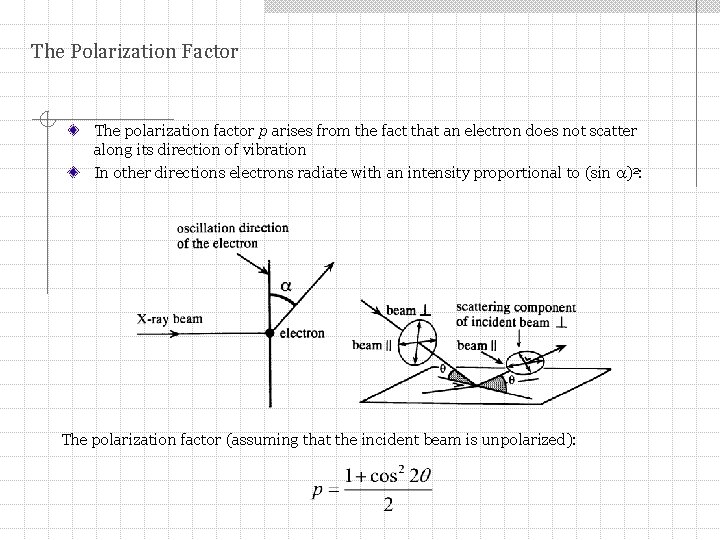
The Polarization Factor The polarization factor p arises from the fact that an electron does not scatter along its direction of vibration In other directions electrons radiate with an intensity proportional to (sin )2: The polarization factor (assuming that the incident beam is unpolarized):

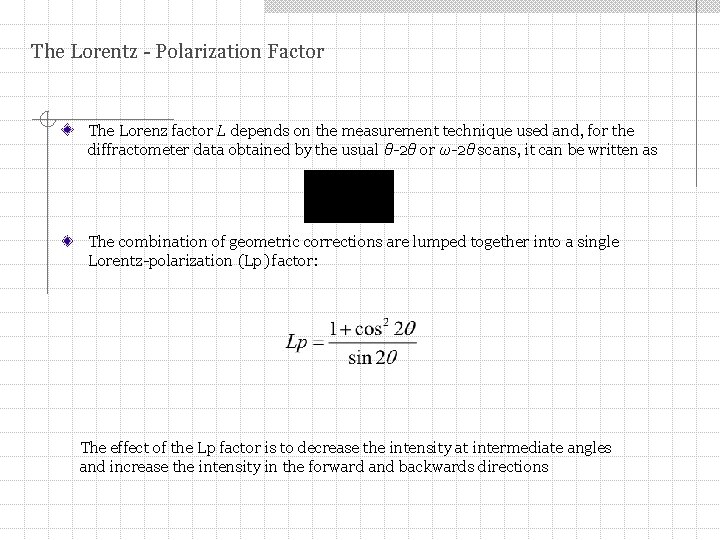
The Lorentz - Polarization Factor The Lorenz factor L depends on the measurement technique used and, for the diffractometer data obtained by the usual θ-2θ or ω-2θ scans, it can be written as The combination of geometric corrections are lumped together into a single Lorentz-polarization (Lp) factor: The effect of the Lp factor is to decrease the intensity at intermediate angles and increase the intensity in the forward and backwards directions

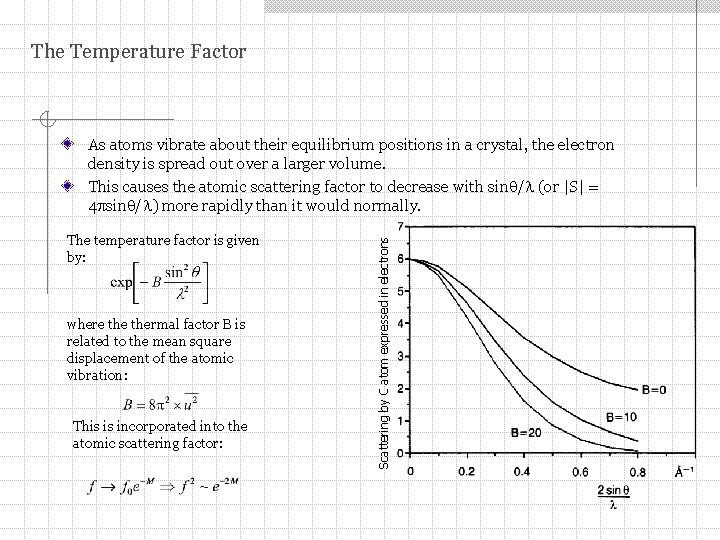
The Temperature Factor The temperature factor is given by: where thermal factor B is related to the mean square displacement of the atomic vibration: This is incorporated into the atomic scattering factor: Scattering by C atom expressed in electrons As atoms vibrate about their equilibrium positions in a crystal, the electron density is spread out over a larger volume. This causes the atomic scattering factor to decrease with sin / (or |S| = 4 psin / ) more rapidly than it would normally.

The Multiplicity Factor The multiplicity factor arises from the fact that in general there will be several sets of hkl -planes having different orientations in a crystal but with the same d and F 2 values Evaluated by finding the number of variations in position and sign in h, k and l and have planes with the same d and F 2 The value depends on hkl and crystal symmetry For the highest cubic symmetry we have: p 100 = 6 p 110 = 12 p 111=8

The Absorption Factor Angle-dependent absorption within the sample itself will modify the observed intensity Absorption factor for infinite thickness specimen is: Absorption factor for thin films is given by: where μ is the absorption coefficient, τ is the total thickness of the film

Diffracted Beam Intensity where K is the scaling factor, Ib is the background intensity, q = 4 sinθ/λ is the scattering vector for x-rays of wavelength λ

Diffraction: Real Samples Up to this point we have been considering diffraction arising from infinitely large crystals that are strain free and behave like ideally imperfect materials ( x-rays only scattered once within a crystal) Crystal size and strain affect the diffraction pattern n we can learn about them from the diffraction pattern High quality crystals such as those produced for the semiconductor industry are not ideally imperfect n need a different theory to understand how they scatter x-rays Not all materials are well ordered crystals

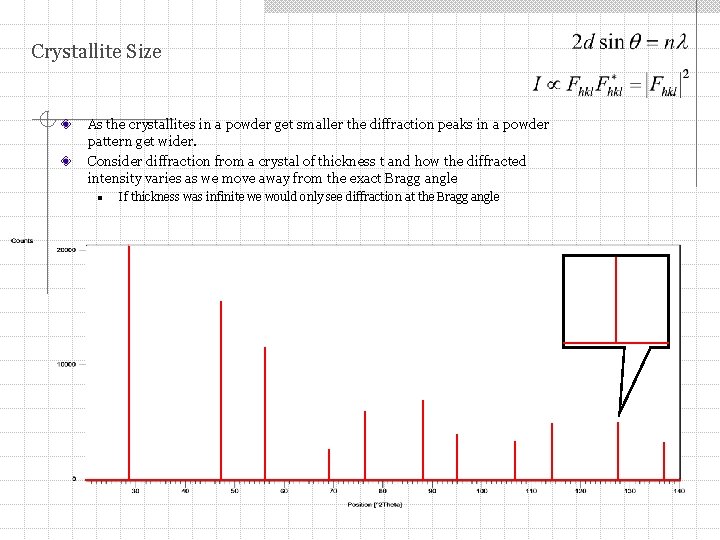
Crystallite Size As the crystallites in a powder get smaller the diffraction peaks in a powder pattern get wider. Consider diffraction from a crystal of thickness t and how the diffracted intensity varies as we move away from the exact Bragg angle n If thickness was infinite we would only see diffraction at the Bragg angle

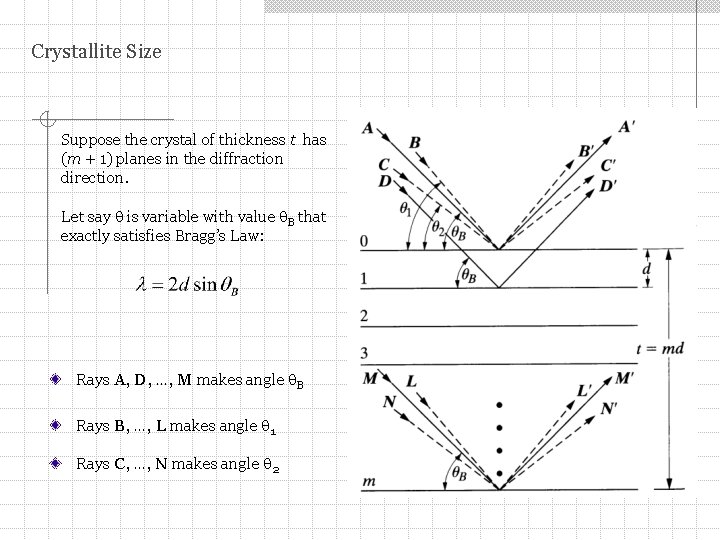
Crystallite Size Suppose the crystal of thickness t has (m + 1) planes in the diffraction direction. Let say is variable with value B that exactly satisfies Bragg’s Law: Rays A, D, …, M makes angle B Rays B, …, L makes angle 1 Rays C, …, N makes angle 2

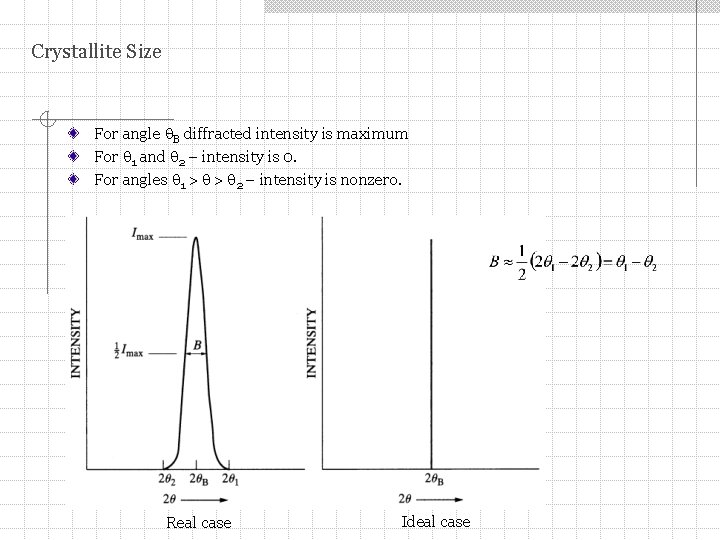
Crystallite Size For angle B diffracted intensity is maximum For 1 and 2 – intensity is 0. For angles 1 > > 2 – intensity is nonzero. Real case Ideal case

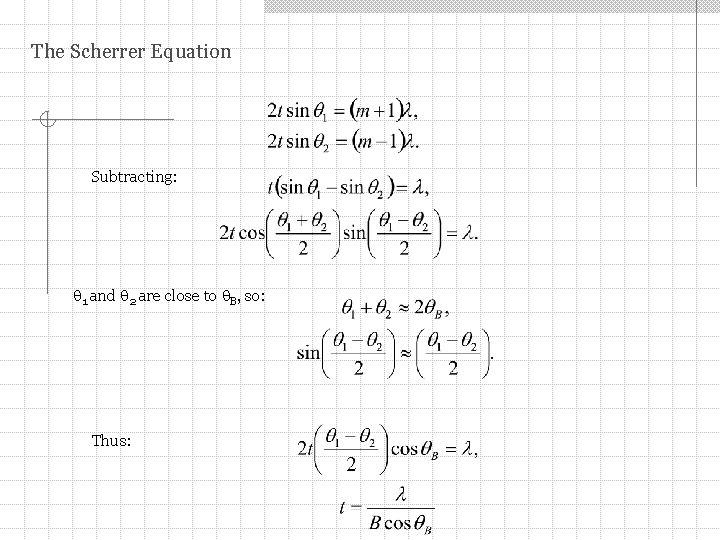
The Scherrer Equation Subtracting: 1 and 2 are close to B, so: Thus:

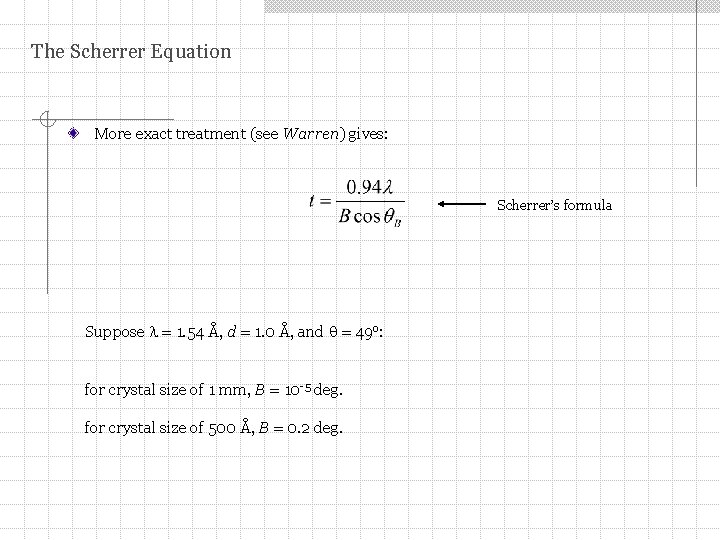
The Scherrer Equation More exact treatment (see Warren) gives: Scherrer’s formula Suppose = 1. 54 Å, d = 1. 0 Å, and = 49 o: for crystal size of 1 mm, B = 10 -5 deg. for crystal size of 500 Å, B = 0. 2 deg.

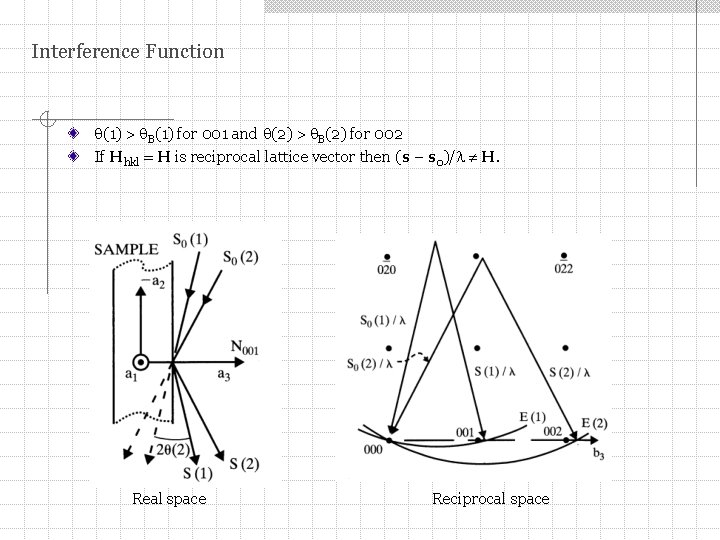
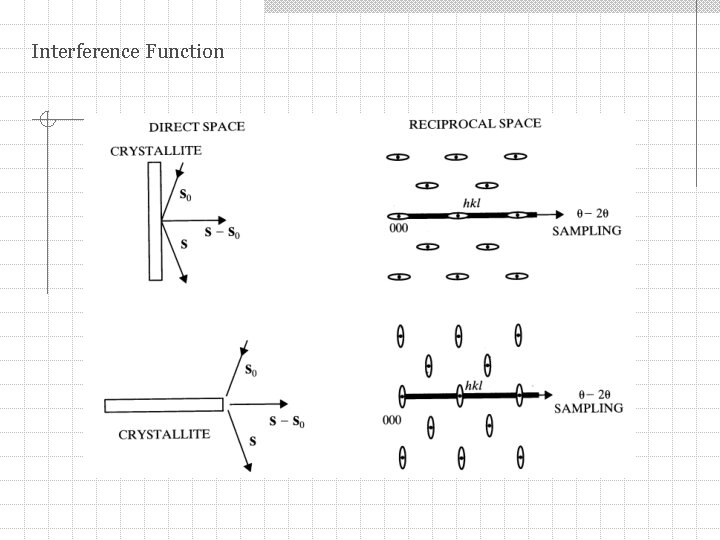
Interference Function We calculate the diffraction peak at the exact Bragg angle B and at angles that have small deviations from B. If crystal is infinite then at B intensity = 0. If crystal is small then at B intensity 0. It varies with angle as a function of the number of unit cells along the diffraction vector (s – s 0). At deviations from B individual unit cells will scatter slightly out of phase. Vector (s – s 0)/ no longer extends to the reciprocal lattice point (RLP).

Interference Function (1) > B(1) for 001 and (2) > B(2) for 002 If Hhkl = H is reciprocal lattice vector then (s – s 0)/ H. Real space Reciprocal space

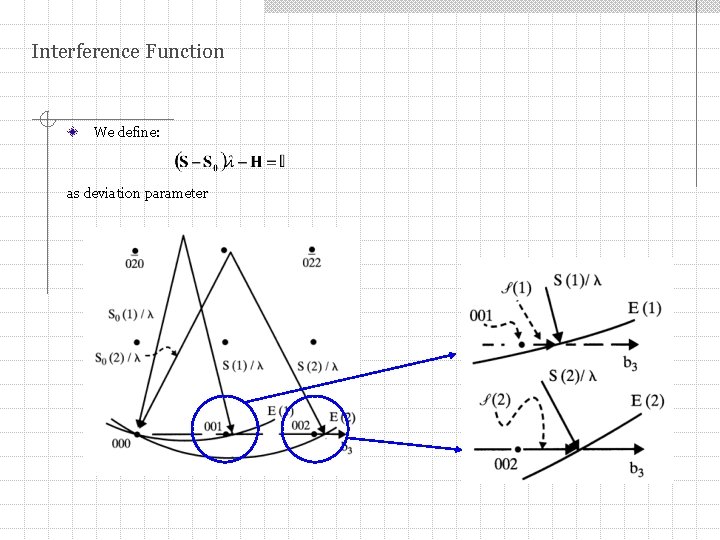
Interference Function We define: as deviation parameter

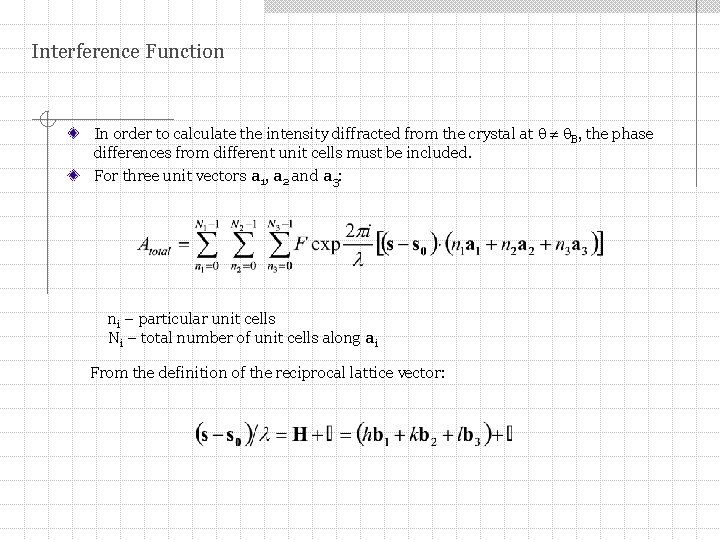
Interference Function In order to calculate the intensity diffracted from the crystal at B, the phase differences from different unit cells must be included. For three unit vectors a 1, a 2 and a 3: ni – particular unit cells Ni – total number of unit cells along ai From the definition of the reciprocal lattice vector:

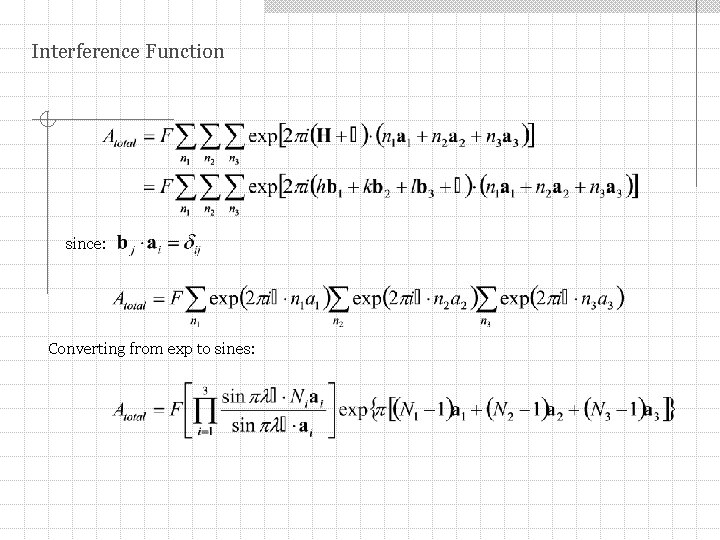
Interference Function since: Converting from exp to sines:

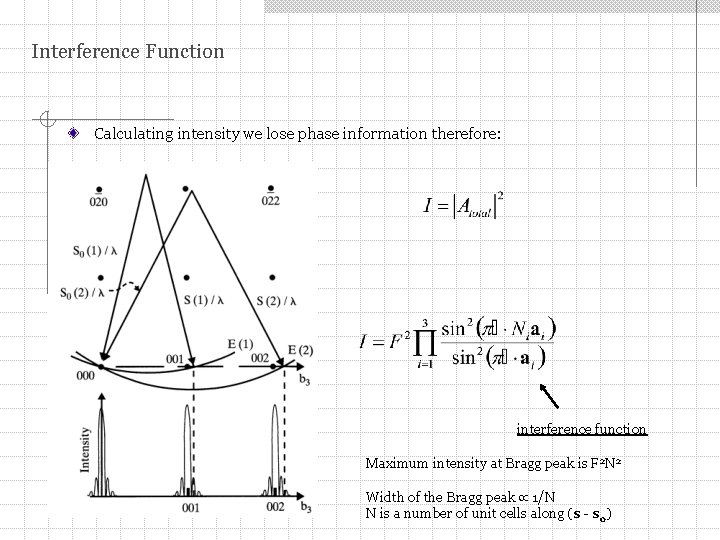
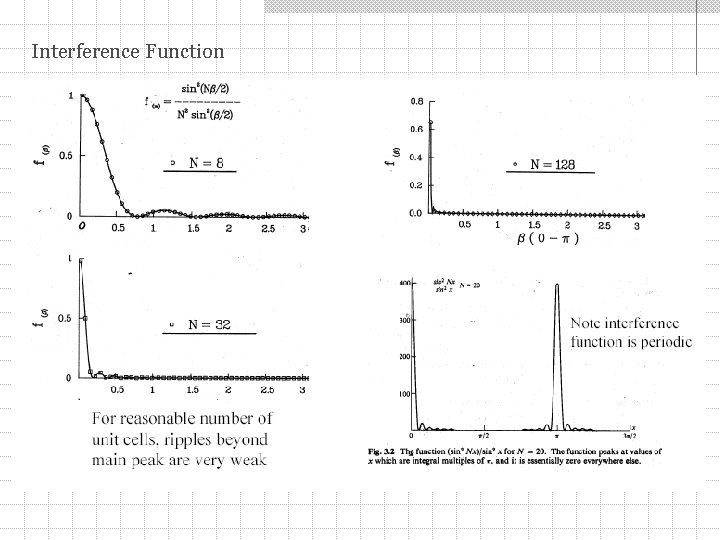
Interference Function Calculating intensity we lose phase information therefore: interference function Maximum intensity at Bragg peak is F 2 N 2 Width of the Bragg peak 1/N N is a number of unit cells along (s - s 0)

Interference Function

Interference Function

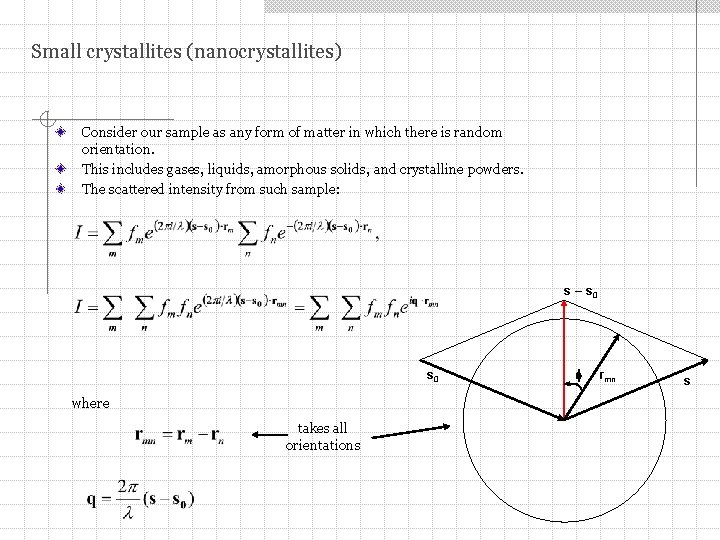
Small crystallites (nanocrystallites) Consider our sample as any form of matter in which there is random orientation. This includes gases, liquids, amorphous solids, and crystalline powders. The scattered intensity from such sample: s – s 0 where takes all orientations f rmn s

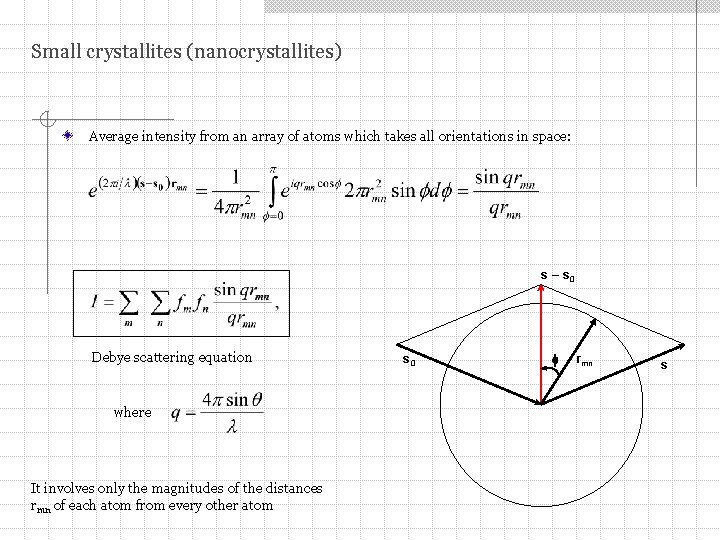
Small crystallites (nanocrystallites) Average intensity from an array of atoms which takes all orientations in space: s – s 0 Debye scattering equation where It involves only the magnitudes of the distances rmn of each atom from every other atom s 0 f rmn s

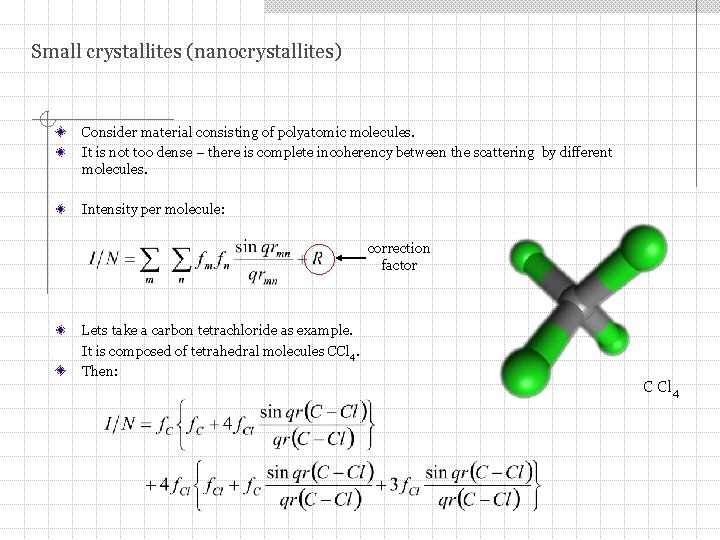
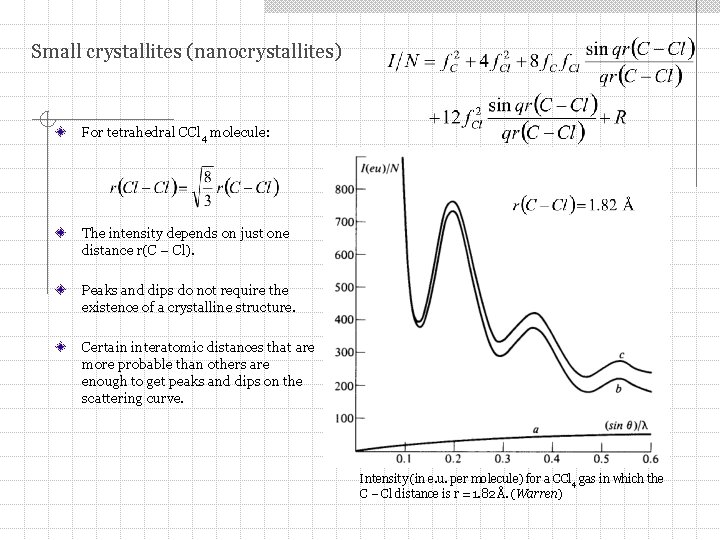
Small crystallites (nanocrystallites) Consider material consisting of polyatomic molecules. It is not too dense – there is complete incoherency between the scattering by different molecules. Intensity per molecule: correction factor Lets take a carbon tetrachloride as example. It is composed of tetrahedral molecules CCl 4. Then: C Cl 4

Small crystallites (nanocrystallites) For tetrahedral CCl 4 molecule: The intensity depends on just one distance r(C – Cl). Peaks and dips do not require the existence of a crystalline structure. Certain interatomic distances that are more probable than others are enough to get peaks and dips on the scattering curve. Intensity (in e. u. per molecule) for a CCl 4 gas in which the C – Cl distance is r = 1. 82 Å. (Warren)

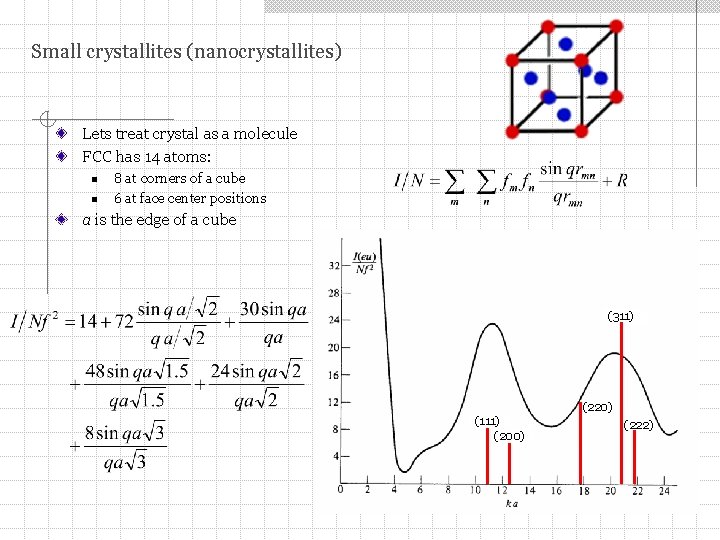
Small crystallites (nanocrystallites) Lets treat crystal as a molecule FCC has 14 atoms: n n 8 at corners of a cube 6 at face center positions a is the edge of a cube (311) (220) (111) (200) (222)

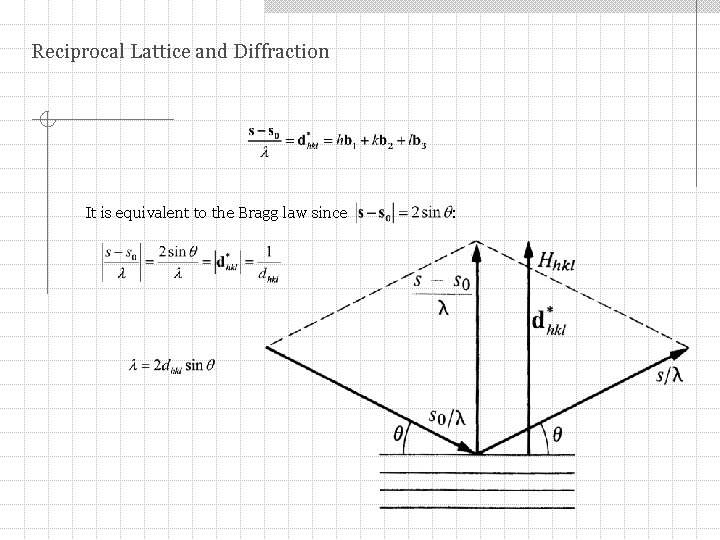
Reciprocal Lattice and Diffraction It is equivalent to the Bragg law since :