XRAY DIFFRACTION XRay Crystallography I XRay Diffraction Uses





- Slides: 22

X-RAY DIFFRACTION (X-Ray Crystallography)

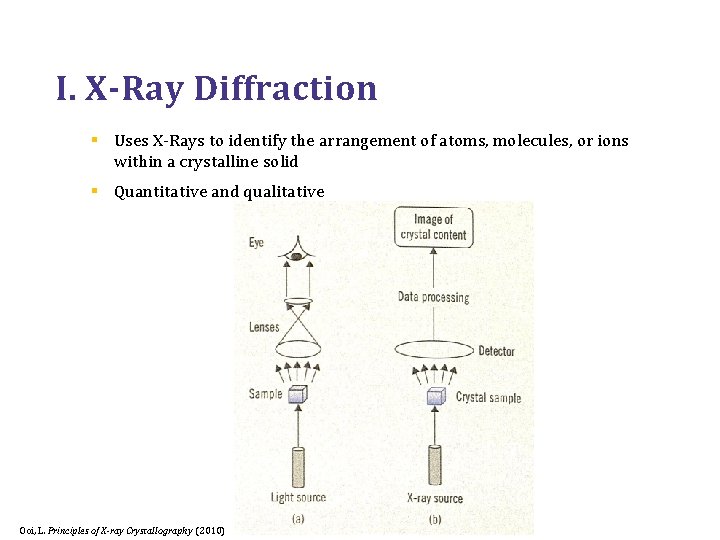
I. X-Ray Diffraction § Uses X-Rays to identify the arrangement of atoms, molecules, or ions within a crystalline solid § Quantitative and qualitative Ooi, L. Principles of X-ray Crystallography (2010)

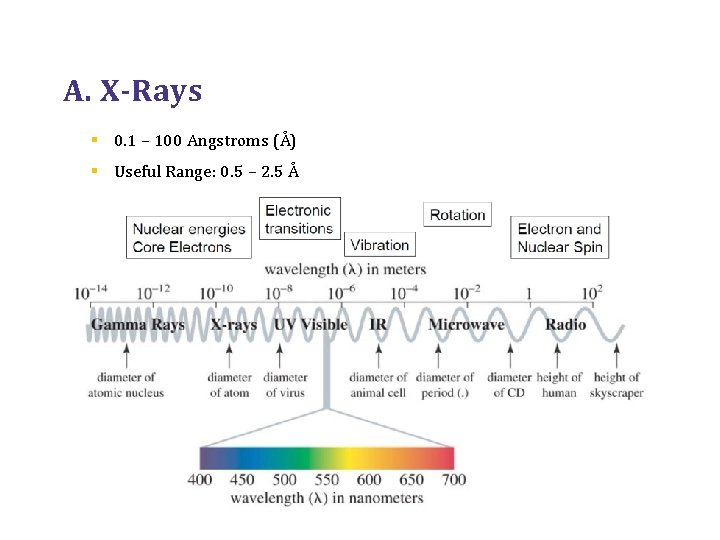
A. X-Rays § 0. 1 – 100 Angstroms (Å) § Useful Range: 0. 5 – 2. 5 Å

B. Amorphous Substances 1. Gases and Liquids § Extremely difficult 2. Non-crystalline Solids § Atoms are not regularly arranged or regularly shaped § Interference § Fiber Diffraction

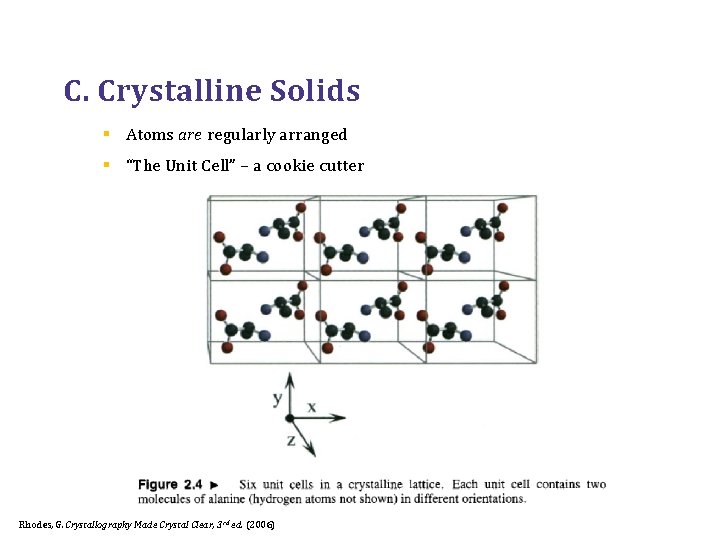
C. Crystalline Solids § Atoms are regularly arranged § “The Unit Cell” – a cookie cutter Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

II. X-Ray Crystallography A. Small-molecule crystallography § Up to ~100 atoms § Organic molecules, catalysts, newly synthesized drugs, etc. § Identify each atom B. Macromolecular (protein) crystallography § Large biological molecules – nucleic acids and proteins § Identify 2° structure Note: must show that the crystal structure (asymmetric unit) is comparable to structure in solution (biological unit)

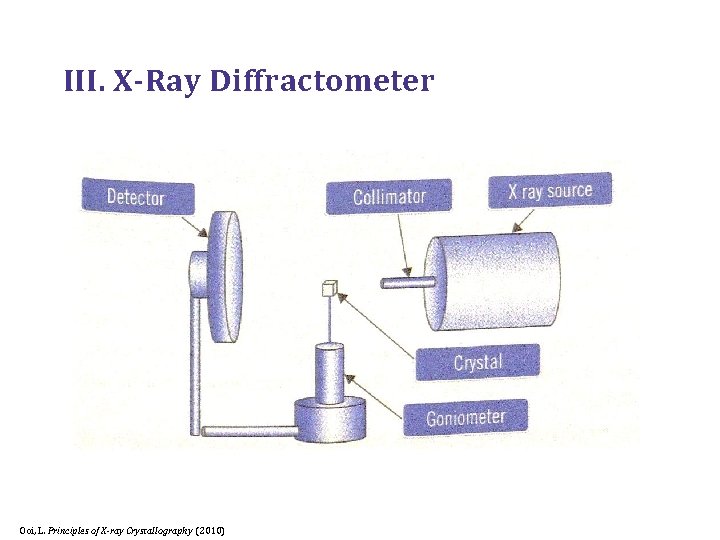
III. X-Ray Diffractometer Ooi, L. Principles of X-ray Crystallography (2010)

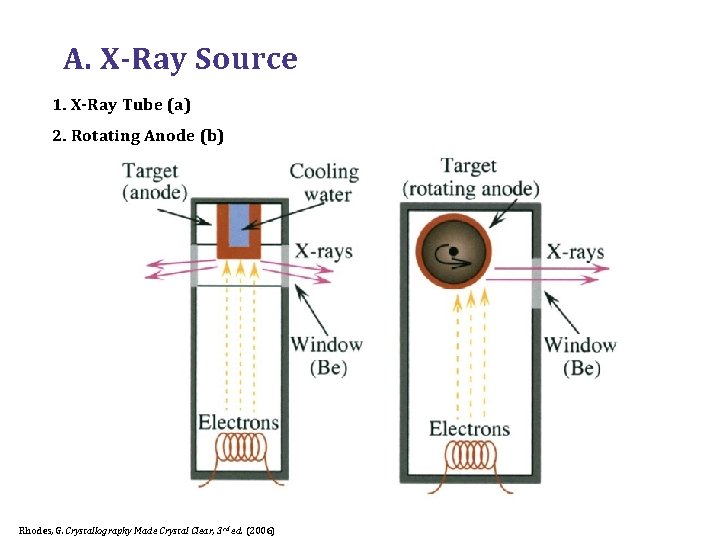
A. X-Ray Source 1. X-Ray Tube (a) 2. Rotating Anode (b) Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

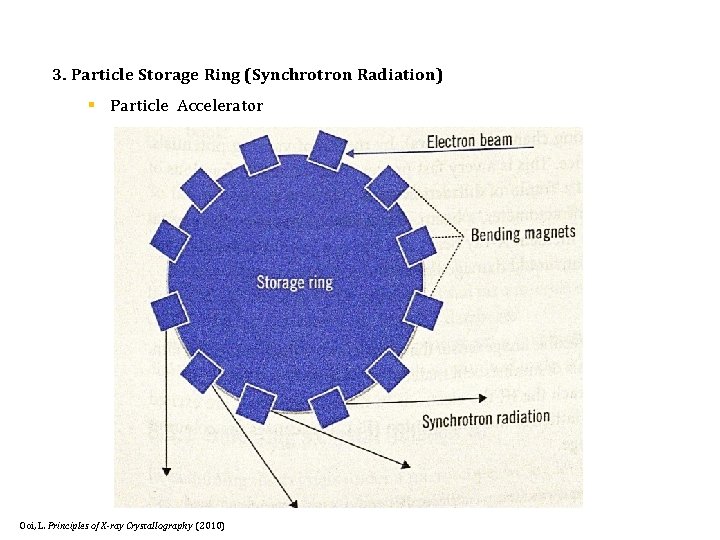
3. Particle Storage Ring (Synchrotron Radiation) § Particle Accelerator Ooi, L. Principles of X-ray Crystallography (2010)

§ National Synchrotron Light Source at Brookhaven National Lab (Long Island) Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

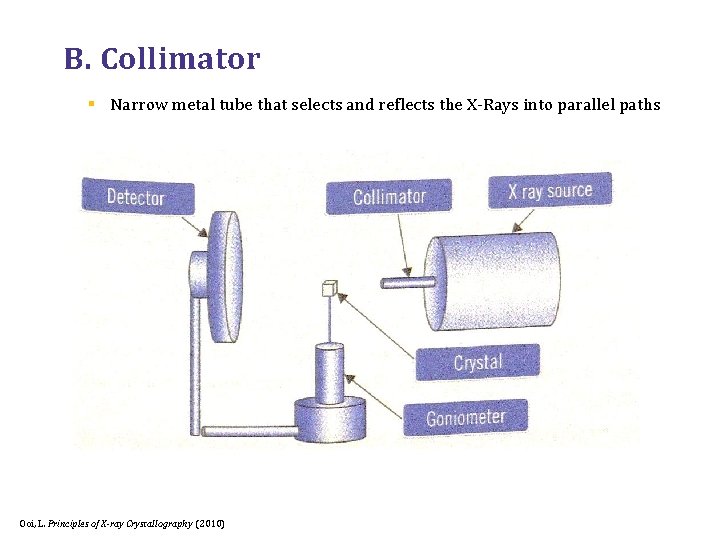
B. Collimator § Narrow metal tube that selects and reflects the X-Rays into parallel paths Ooi, L. Principles of X-ray Crystallography (2010)

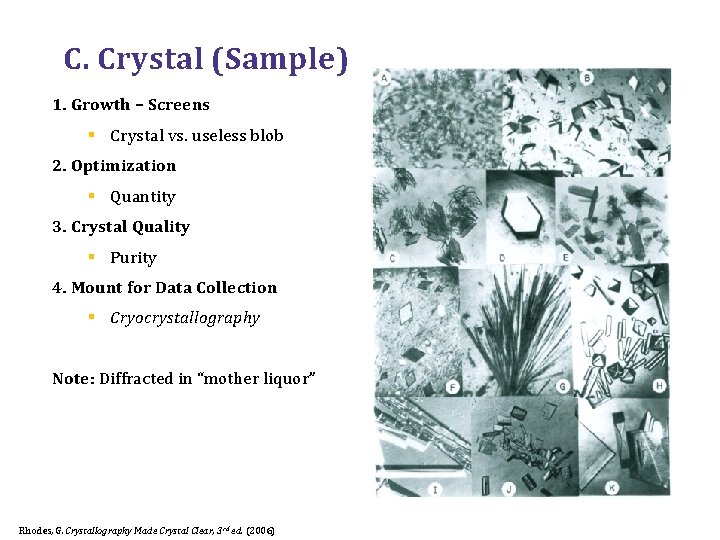
C. Crystal (Sample) 1. Growth – Screens § Crystal vs. useless blob 2. Optimization § Quantity 3. Crystal Quality § Purity 4. Mount for Data Collection § Cryocrystallography Note: Diffracted in “mother liquor” Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

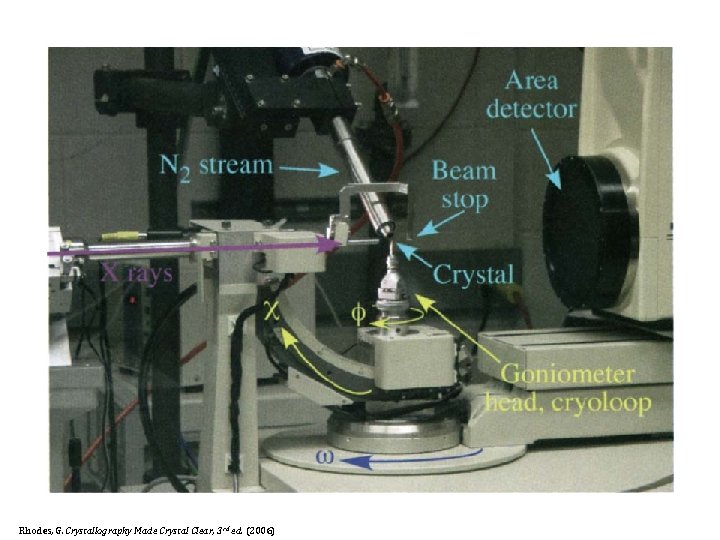
D. Camera § Goniometer § Goniostat Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

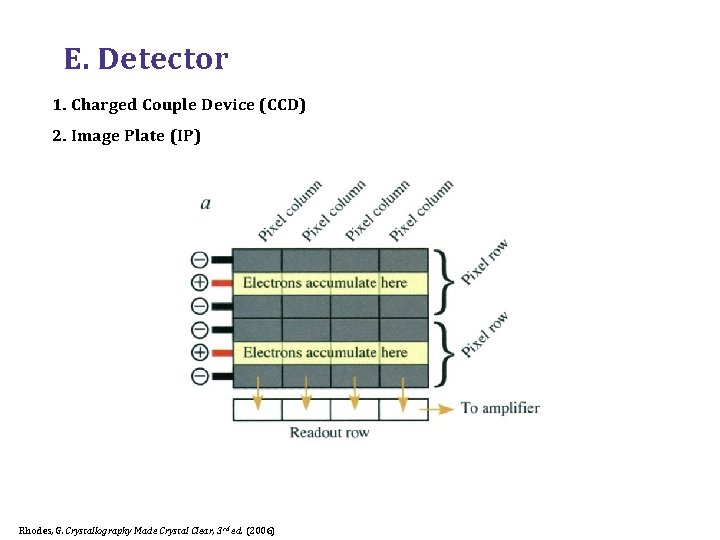
E. Detector 1. Charged Couple Device (CCD) 2. Image Plate (IP) Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

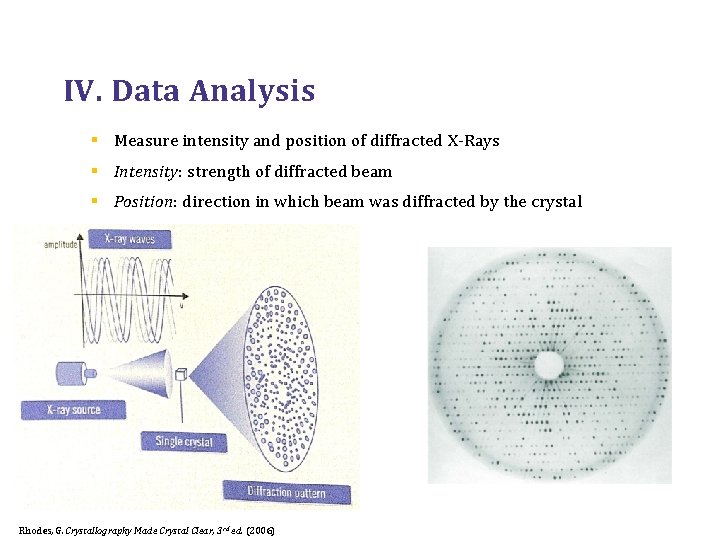
IV. Data Analysis § Measure intensity and position of diffracted X-Rays § Intensity: strength of diffracted beam § Position: direction in which beam was diffracted by the crystal Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

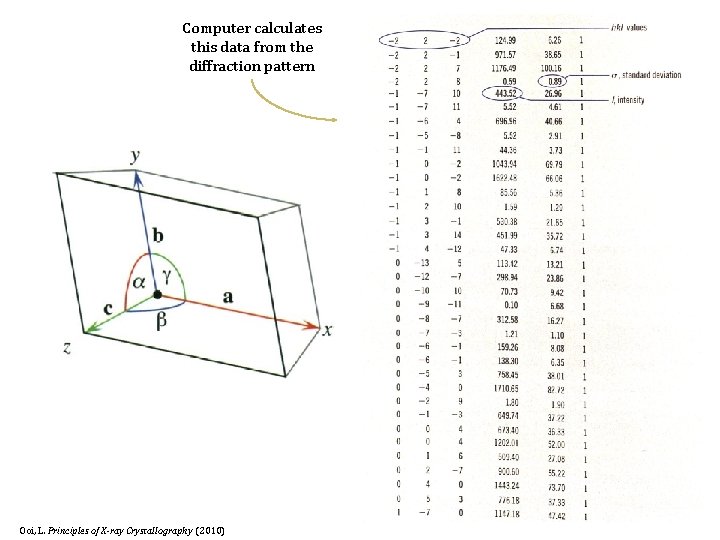
Computer calculates this data from the diffraction pattern Ooi, L. Principles of X-ray Crystallography (2010)

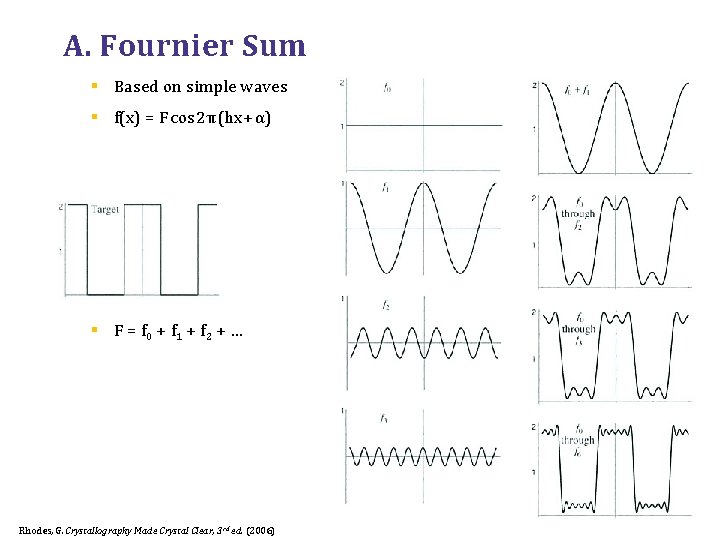
A. Fournier Sum § Based on simple waves § f(x) = F cos 2π (hx + α) § F = f 0 + f 1 + f 2 + … Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)

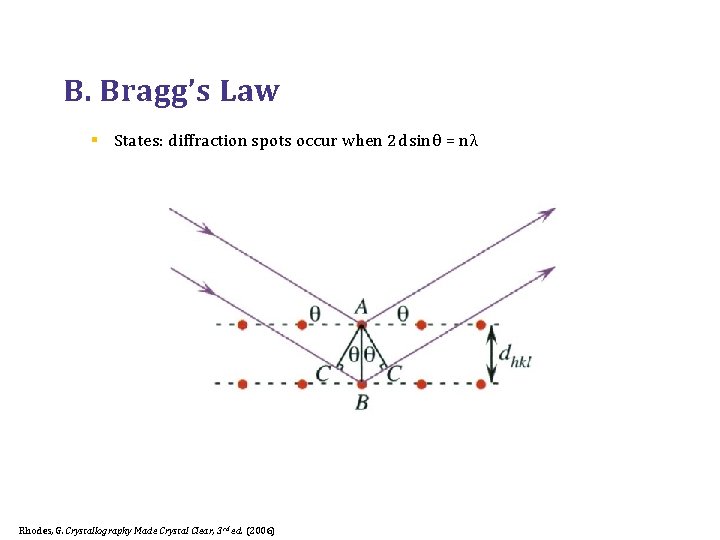
B. Bragg’s Law § States: diffraction spots occur when 2 d sin θ = n λ Rhodes, G. Crystallography Made Crystal Clear, 3 rd ed. (2006)



Benefits § Molecular structure in solid crystalline state with extreme certainty § Direct inference of data § Provides limitless info. Downfalls § Crystals § Slow § Hydrogen § Still just a model