XRay Diffraction The XRD Technique Takes a sample





- Slides: 16

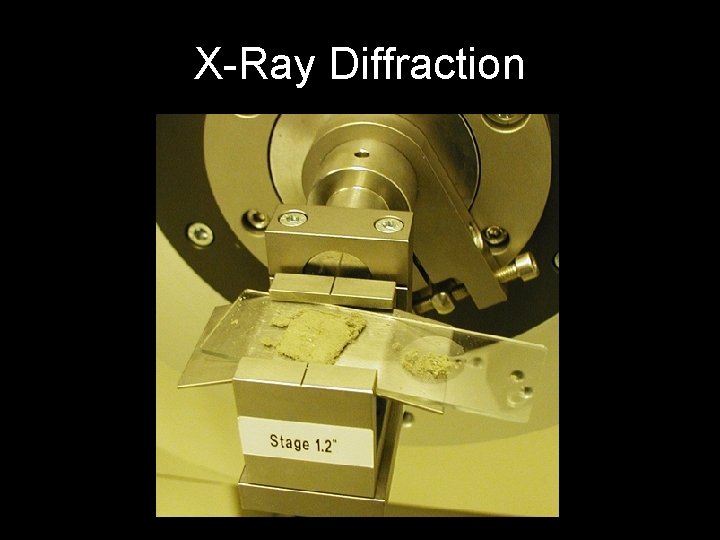
X-Ray Diffraction

The XRD Technique • Takes a sample of the material and places a powdered sample which is then illuminated with x-rays of a fixed wave-length. • The intensity of the reflected radiation is recorded using a goniometer. • The data is analyzed for the reflection angle to calculate the inter-atomic spacing. • The intensity is measured to discriminate the various D spacing and the results are compared to known data to identify possible matches.

Powdering Samples • The samples are powdered to give a random sampling of ALL atomic planes (crystal faces) • Statistically accurate given samples are powdered finely AND randomly oriented on sample holder – Intensities are a reflection of d-spacing abundance • Problems arise with minerals that may preferentially orient on sample holder – Micas and clays have special preparation techniques

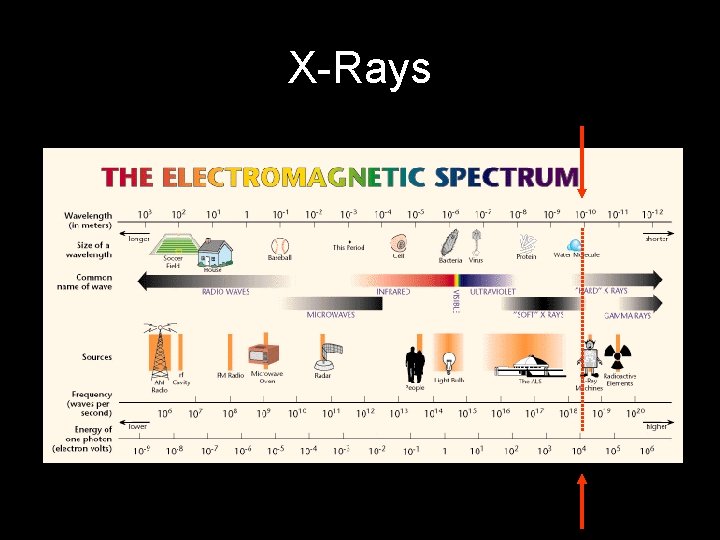
X-Rays Wavelengths used for XRD

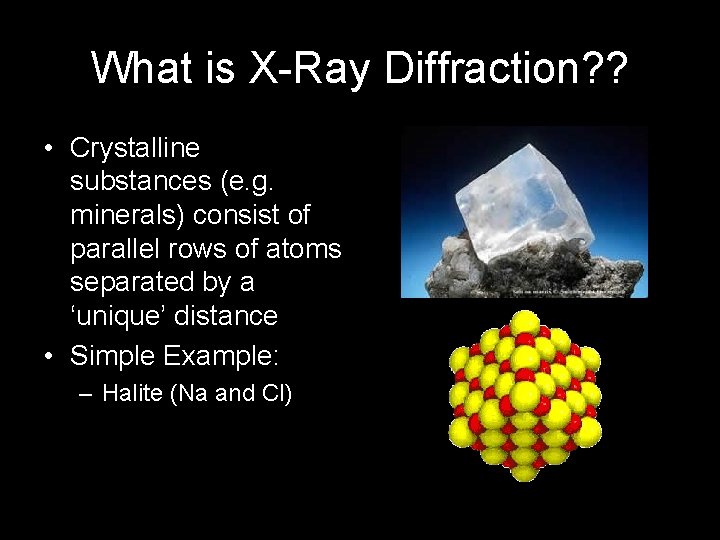
What is X-Ray Diffraction? ? • Crystalline substances (e. g. minerals) consist of parallel rows of atoms separated by a ‘unique’ distance • Simple Example: – Halite (Na and Cl)

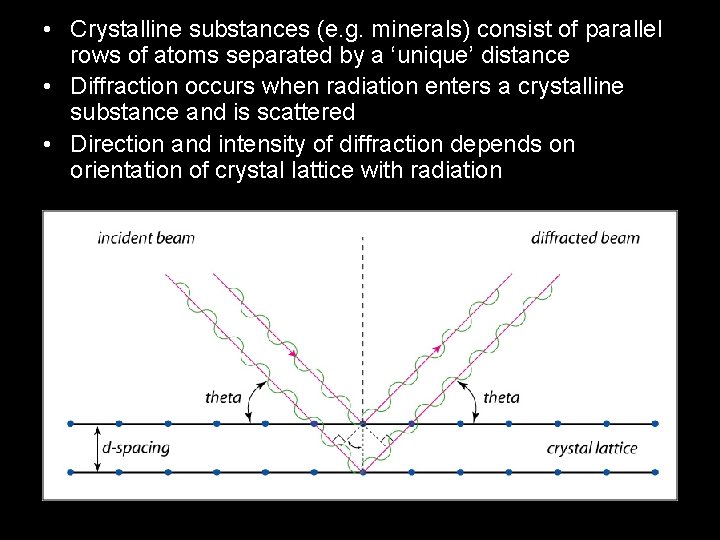
• Crystalline substances (e. g. minerals) consist of parallel rows of atoms separated by a ‘unique’ distance • Diffraction occurs when radiation enters a crystalline substance and is scattered • Direction and intensity of diffraction depends on orientation of crystal lattice with radiation

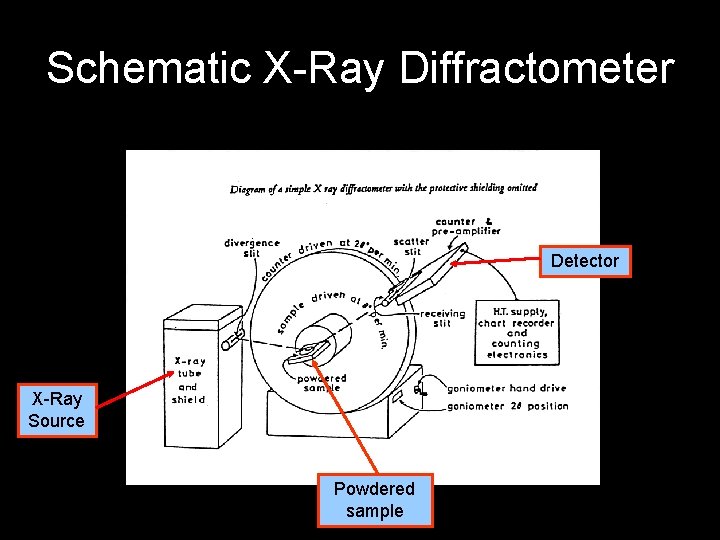
Schematic X-Ray Diffractometer Detector X-Ray Source Powdered sample

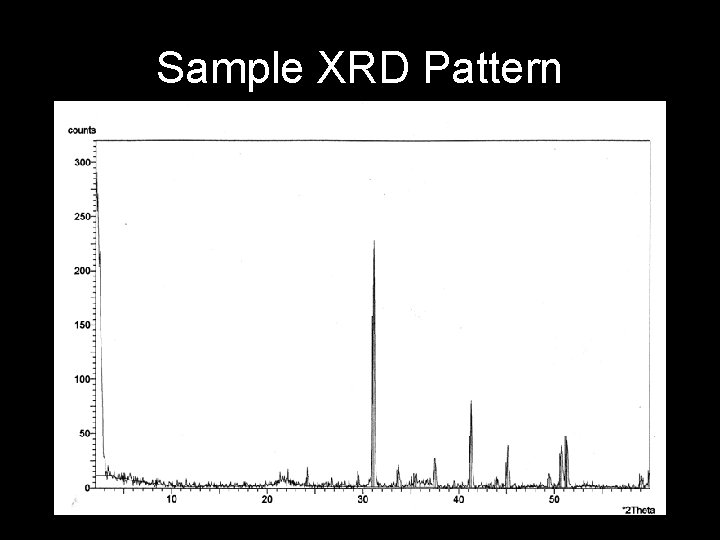
Sample XRD Pattern

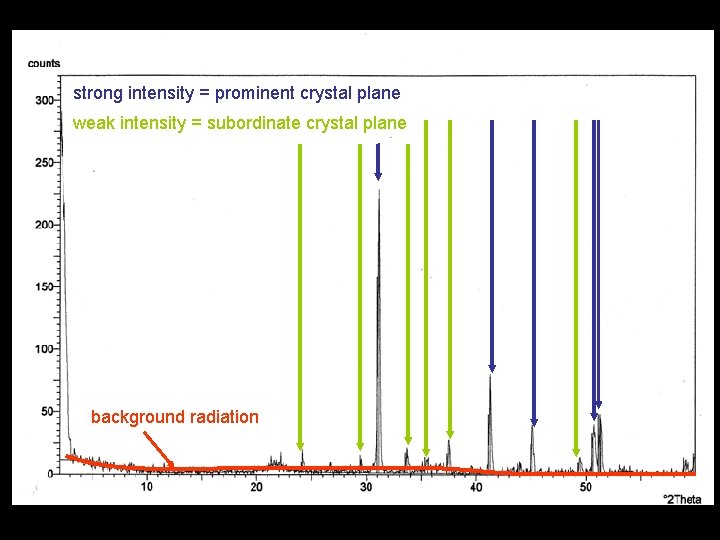
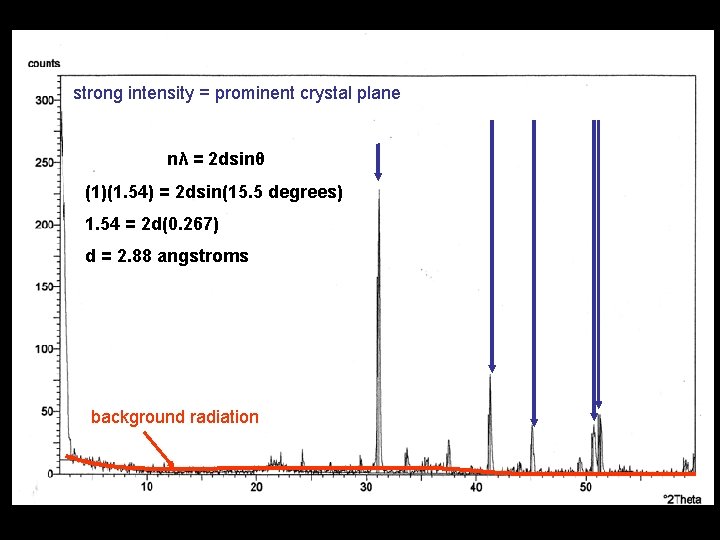
strong intensity = prominent crystal plane weak intensity = subordinate crystal plane background radiation

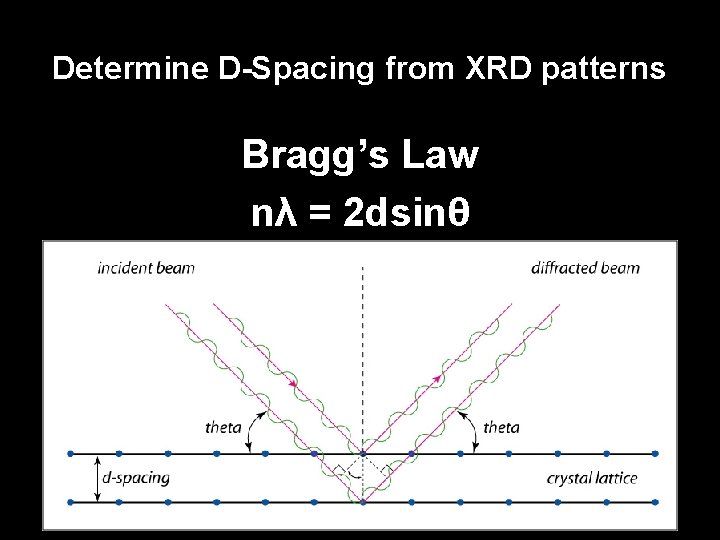
Determine D-Spacing from XRD patterns Bragg’s Law nλ = 2 dsinθ • n = reflection order (1, 2, 3, 4, etc…) • λ = radiation wavelength (1. 54 angstroms) • d = spacing between planes of atoms (angstroms) • θ = angle of incidence (degrees)

strong intensity = prominent crystal plane nλ = 2 dsinθ (1)(1. 54) = 2 dsin(15. 5 degrees) 1. 54 = 2 d(0. 267) d = 2. 88 angstroms background radiation

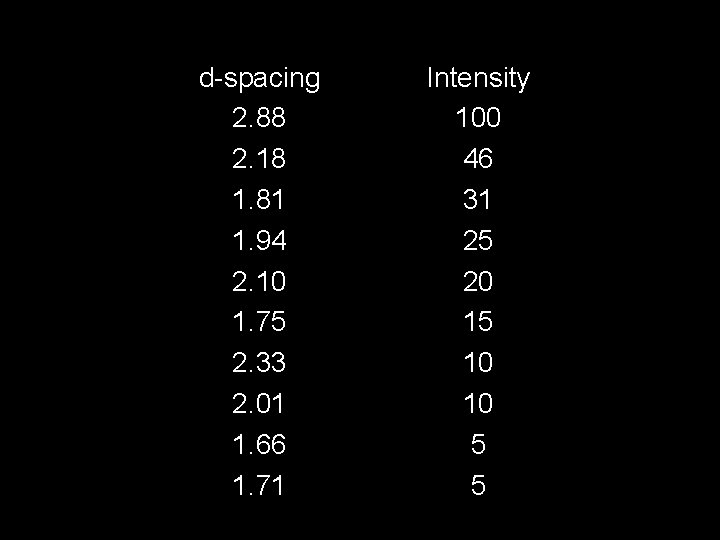
d-spacing 2. 88 2. 18 1. 81 1. 94 2. 10 1. 75 2. 33 2. 01 1. 66 1. 71 Intensity 100 46 31 25 20 15 10 10 5 5

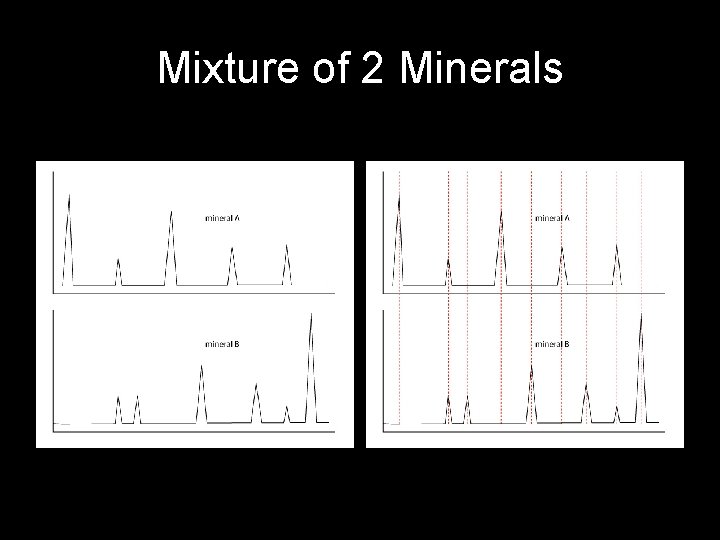
Factors that affect XRD data • Sample not powdered fine enough – May not give all d-spacing data (not random enough) • Analysis too fast (degrees/minute) – May not give accurate peak data • Mixture of minerals? ? • Not crystalline – glass!!

Mixture of 2 Minerals

Applications of XRD • • Unknown mineral ID Solid solution ID (e. g. feldspars, olivine) Mixtures of minerals Clay analyses Zeolites Crystallographic applications Material Science

Created by Nicolas Barth 2007 Geology 114 A University of California, Santa Barbara Source material by Grant Yip