Xp v Y Xpp v Yp v v
![Fig 7. 3 The bacteria flagella motor [source: Berg HC, Ann. Rev. Biochem 2003] Fig 7. 3 The bacteria flagella motor [source: Berg HC, Ann. Rev. Biochem 2003]](https://slidetodoc.com/presentation_image_h/80cf1540484dd59ba7967c62f6b7d826/image-19.jpg)
![Tumbling frequency 1/sec Attractant added exact adaptation Time [min] Fig 7. 5 : Average Tumbling frequency 1/sec Attractant added exact adaptation Time [min] Fig 7. 5 : Average](https://slidetodoc.com/presentation_image_h/80cf1540484dd59ba7967c62f6b7d826/image-21.jpg)
![Fig 7. 6 The chemotaxis signal transduction network [source: Alon et al Nature 1999] Fig 7. 6 The chemotaxis signal transduction network [source: Alon et al Nature 1999]](https://slidetodoc.com/presentation_image_h/80cf1540484dd59ba7967c62f6b7d826/image-22.jpg)



















- Slides: 71

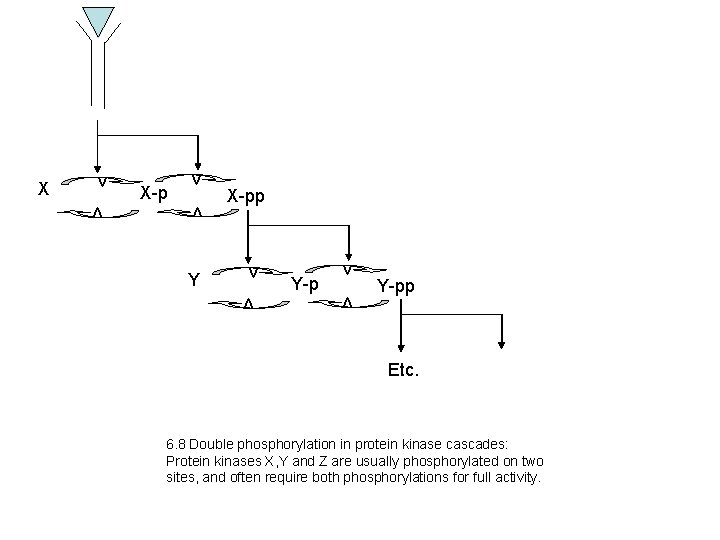
X-p v Y X-pp v Y-p v v v X Y-pp Etc. 6. 8 Double phosphorylation in protein kinase cascades: Protein kinases X, Y and Z are usually phosphorylated on two sites, and often require both phosphorylations for full activity.

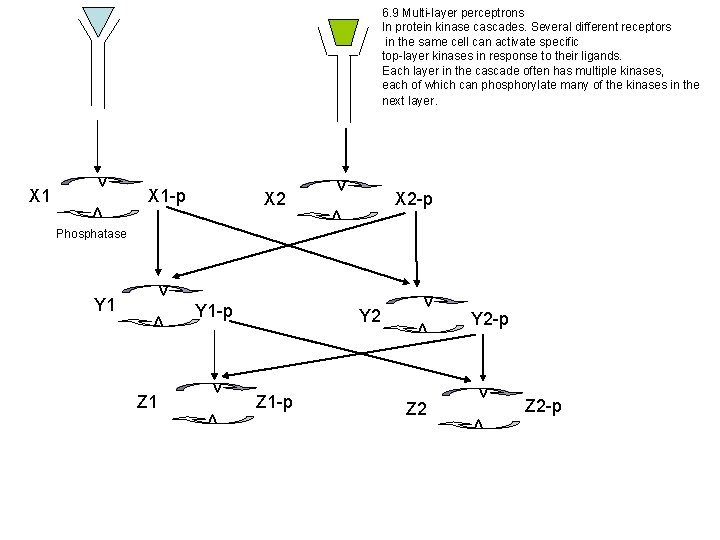
6. 9 Multi-layer perceptrons In protein kinase cascades. Several different receptors in the same cell can activate specific top-layer kinases in response to their ligands. Each layer in the cascade often has multiple kinases, each of which can phosphorylate many of the kinases in the next layer. X 1 -p X 2 v X 2 -p v v Phosphatase v v Z 1 Y 1 -p Y 2 Z 1 -p v Z 2 Y 2 -p v v v Y 1 v v X 1 v Z 2 -p

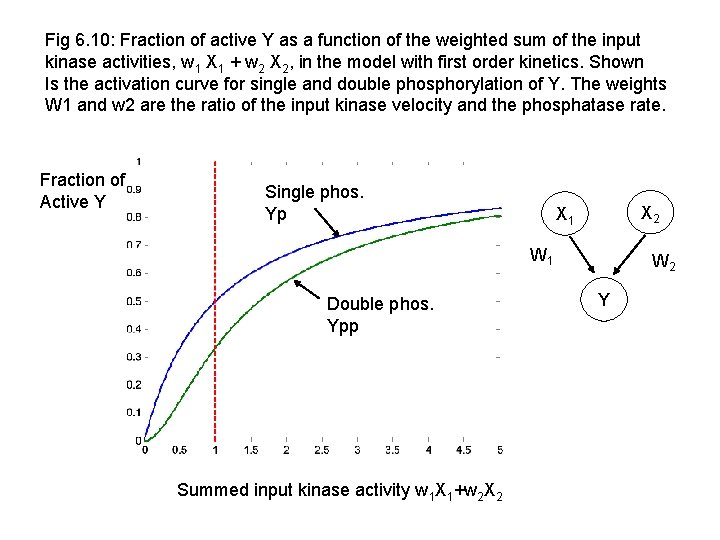
Fig 6. 10: Fraction of active Y as a function of the weighted sum of the input kinase activities, w 1 X 1 + w 2 X 2, in the model with first order kinetics. Shown Is the activation curve for single and double phosphorylation of Y. The weights W 1 and w 2 are the ratio of the input kinase velocity and the phosphatase rate. Fraction of Active Y Single phos. Yp X 2 X 1 W 1 Double phos. Ypp Summed input kinase activity w 1 X 1+w 2 X 2 W 2 Y

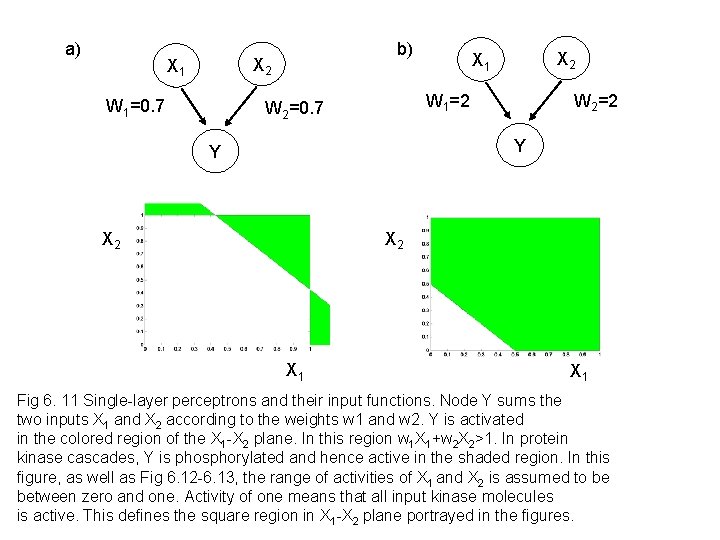
a) b) X 2 X 1 W 1=0. 7 X 2 X 1 W 1=2 W 2=0. 7 W 2=2 Y Y X 2 X 1 Fig 6. 11 Single-layer perceptrons and their input functions. Node Y sums the two inputs X 1 and X 2 according to the weights w 1 and w 2. Y is activated in the colored region of the X 1 -X 2 plane. In this region w 1 X 1+w 2 X 2>1. In protein kinase cascades, Y is phosphorylated and hence active in the shaded region. In this figure, as well as Fig 6. 12 -6. 13, the range of activities of X 1 and X 2 is assumed to be between zero and one. Activity of one means that all input kinase molecules is active. This defines the square region in X 1 -X 2 plane portrayed in the figures.

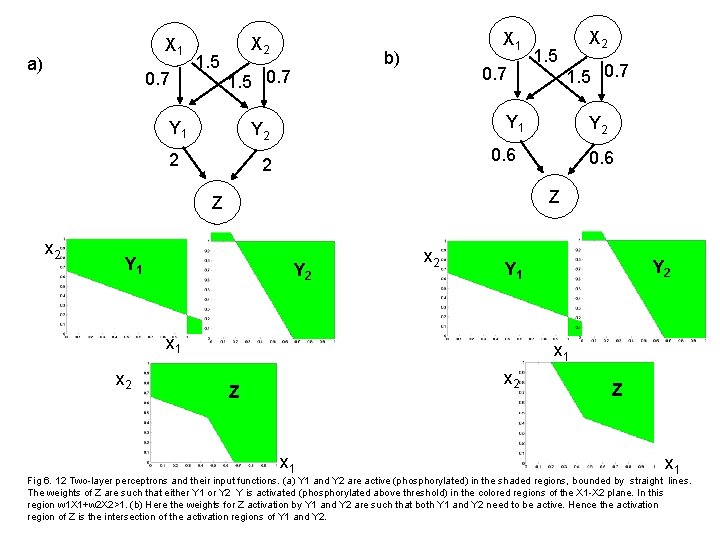
X 1 a) 0. 7 1. 5 X 2 b) 1. 5 0. 7 Y 1 X 1 0. 7 0. 6 Z Y 1 Y 2 x 2 Y 1 x 2 Y 2 0. 6 2 Z x 2 1. 5 0. 7 Y 1 Y 2 2 1. 5 X 2 x 1 x 2 Z x 1 Fig 6. 12 Two-layer perceptrons and their input functions. (a) Y 1 and Y 2 are active (phosphorylated) in the shaded regions, bounded by straight lines. The weights of Z are such that either Y 1 or Y 2 Y is activated (phosphorylated above threshold) in the colored regions of the X 1 -X 2 plane. In this region w 1 X 1+w 2 X 2>1. (b) Here the weights for Z activation by Y 1 and Y 2 are such that both Y 1 and Y 2 need to be active. Hence the activation region of Z is the intersection of the activation regions of Y 1 and Y 2.

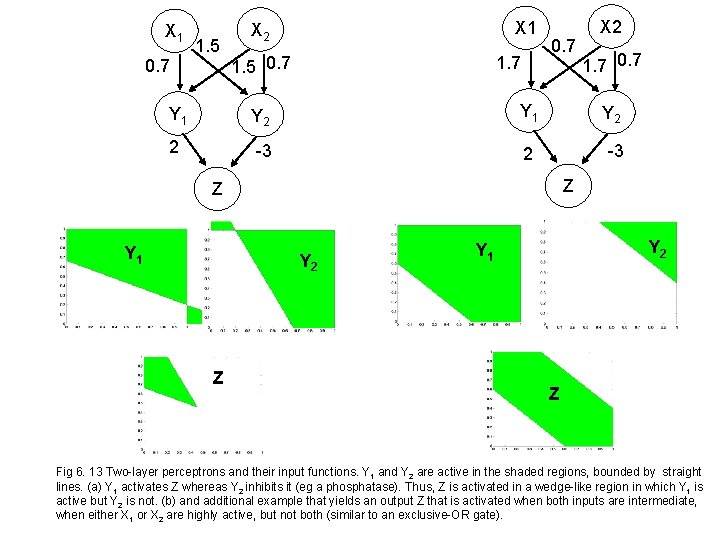
X 1 0. 7 1. 5 X 1 X 2 1. 7 1. 5 0. 7 X 2 1. 7 0. 7 Y 1 Y 2 2 -3 Z Z Y 1 Y 2 Z Y 2 Y 1 Z Fig 6. 13 Two-layer perceptrons and their input functions. Y 1 and Y 2 are active in the shaded regions, bounded by straight lines. (a) Y 1 activates Z whereas Y 2 inhibits it (eg a phosphatase). Thus, Z is activated in a wedge-like region in which Y 1 is active but Y 2 is not. (b) and additional example that yields an output Z that is activated when both inputs are intermediate, when either X 1 or X 2 are highly active, but not both (similar to an exclusive-OR gate).

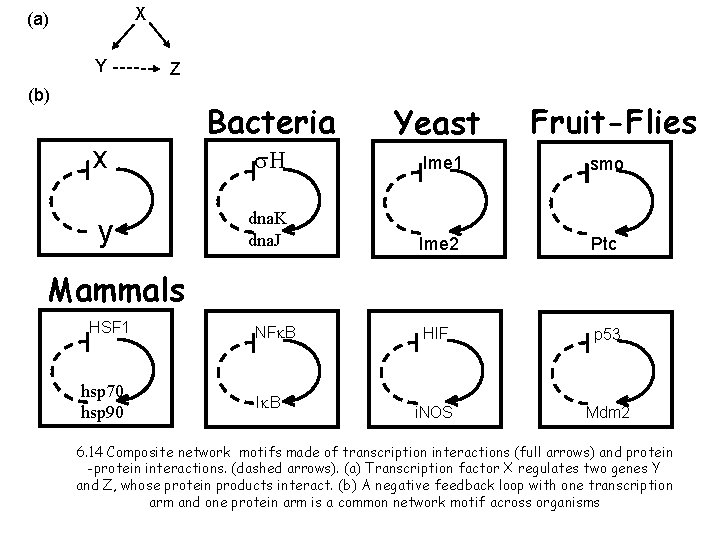
X (a) Y Z (b) x y Bacteria Yeast Fruit-Flies s. H Ime 1 smo dna. K dna. J Ime 2 Ptc HIF p 53 i. NOS Mdm 2 Mammals HSF 1 hsp 70 hsp 90 NFk. B Ik. B 6. 14 Composite network motifs made of transcription interactions (full arrows) and protein -protein interactions. (dashed arrows). (a) Transcription factor X regulates two genes Y and Z, whose protein products interact. (b) A negative feedback loop with one transcription arm and one protein arm is a common network motif across organisms

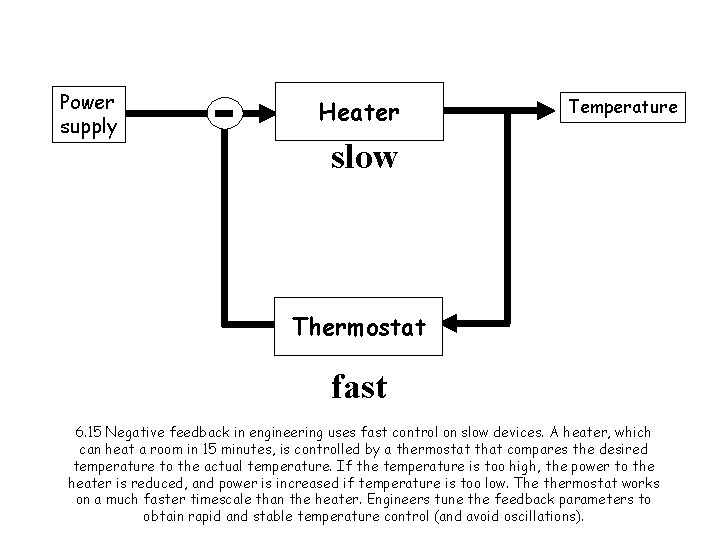
Power supply Heater Temperature slow Thermostat fast 6. 15 Negative feedback in engineering uses fast control on slow devices. A heater, which can heat a room in 15 minutes, is controlled by a thermostat that compares the desired temperature to the actual temperature. If the temperature is too high, the power to the heater is reduced, and power is increased if temperature is too low. The thermostat works on a much faster timescale than the heater. Engineers tune the feedback parameters to obtain rapid and stable temperature control (and avoid oscillations).

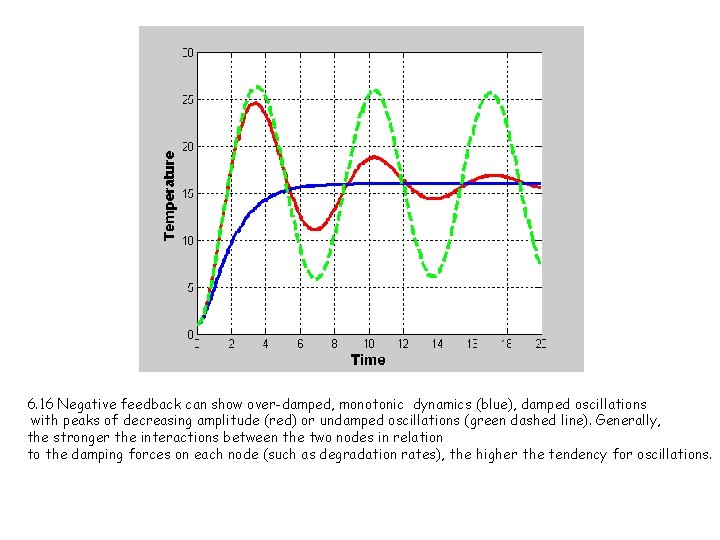
6. 16 Negative feedback can show over-damped, monotonic dynamics (blue), damped oscillations with peaks of decreasing amplitude (red) or undamped oscillations (green dashed line). Generally, the stronger the interactions between the two nodes in relation to the damping forces on each node (such as degradation rates), the higher the tendency for oscillations.

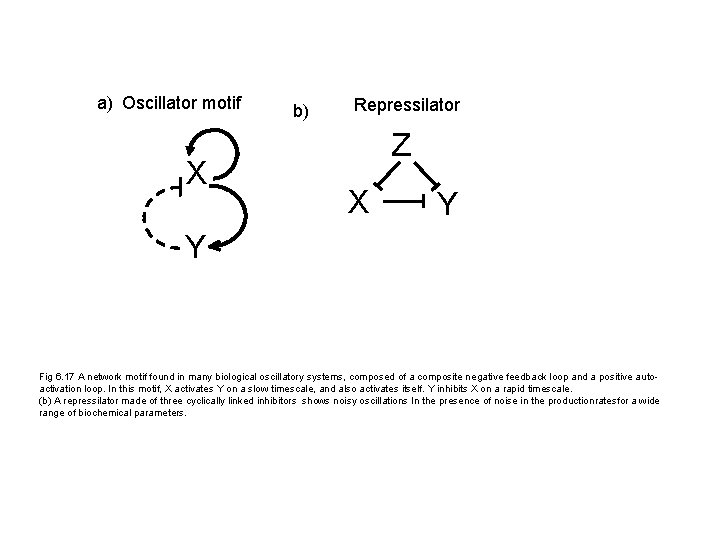
a) Oscillator motif X b) Repressilator Z X Y Y Fig 6. 17 A network motif found in many biological oscillatory systems, composed of a composite negative feedback loop and a positive autoactivation loop. In this motif, X activates Y on a slow timescale, and also activates itself. Y inhibits X on a rapid timescale. (b) A repressilator made of three cyclically linked inhibitors shows noisy oscillations In the presence of noise in the productionratesfor a wide range of biochemical parameters.

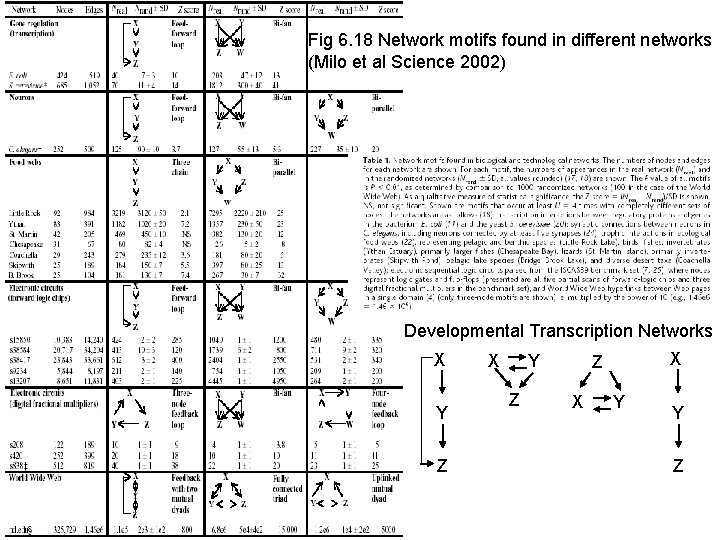
Fig 6. 18 Network motifs found in different networks (Milo et al Science 2002) Developmental Transcription Networks X Y Z X Z X Y Y Z

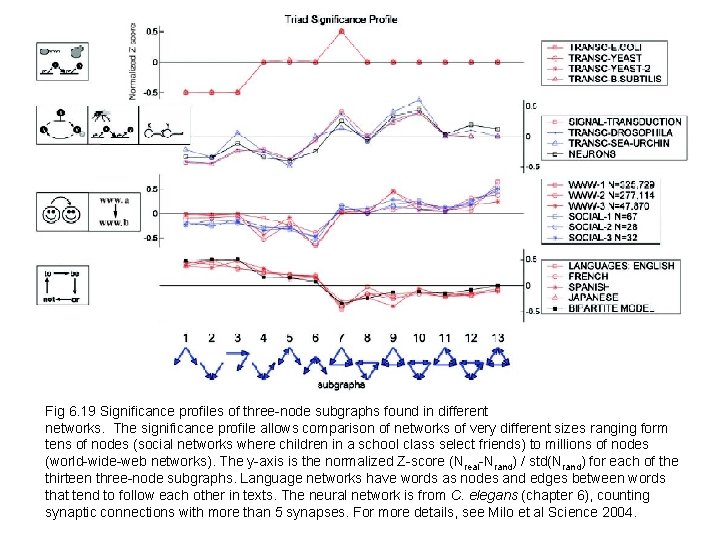
Fig 6. 19 Significance profiles of three-node subgraphs found in different networks. The significance profile allows comparison of networks of very different sizes ranging form tens of nodes (social networks where children in a school class select friends) to millions of nodes (world-wide-web networks). The y-axis is the normalized Z-score (Nreal-Nrand) / std(Nrand) for each of the thirteen three-node subgraphs. Language networks have words as nodes and edges between words that tend to follow each other in texts. The neural network is from C. elegans (chapter 6), counting synaptic connections with more than 5 synapses. For more details, see Milo et al Science 2004.

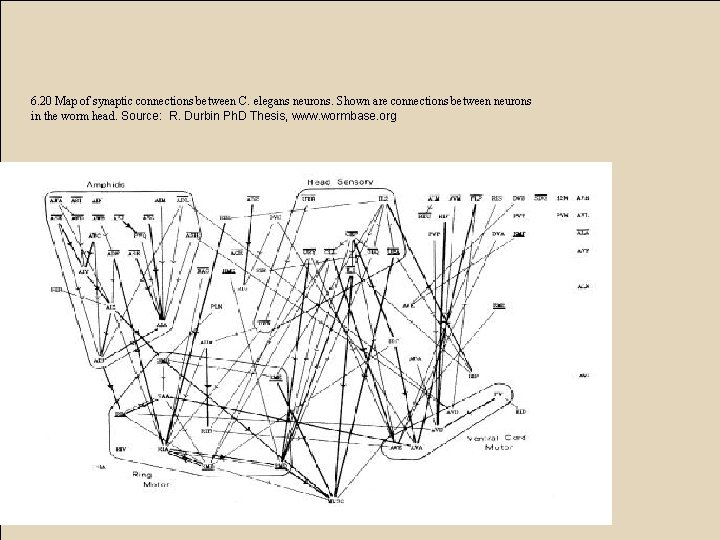
6. 20 Map of synaptic connections between C. elegans neurons. Shown are connections between neurons in the worm head. Source: R. Durbin Ph. D Thesis, www. wormbase. org

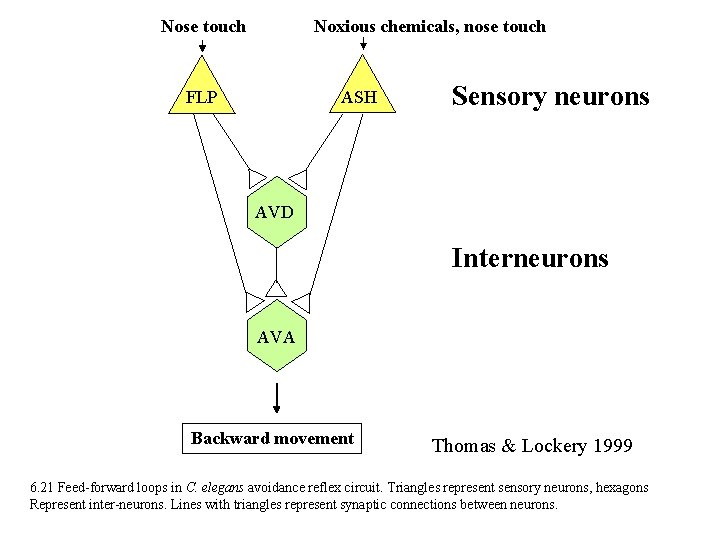
Nose touch Noxious chemicals, nose touch ASH FLP Sensory neurons AVD Interneurons AVA Backward movement Thomas & Lockery 1999 6. 21 Feed-forward loops in C. elegans avoidance reflex circuit. Triangles represent sensory neurons, hexagons Represent inter-neurons. Lines with triangles represent synaptic connections between neurons.

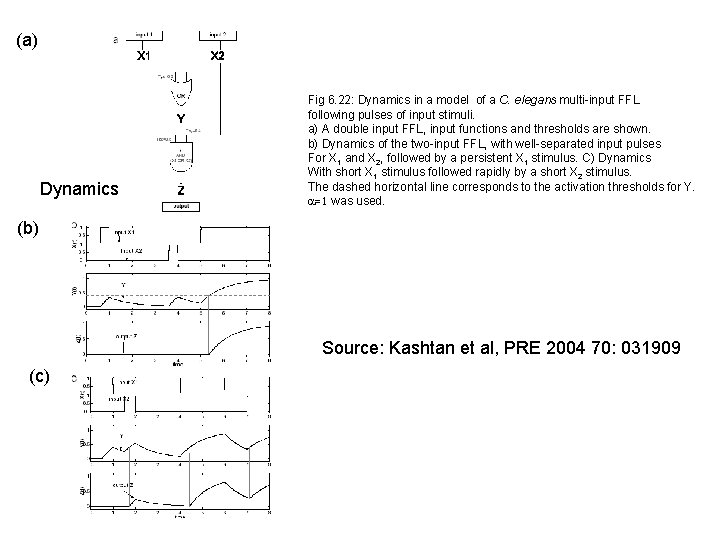
(a) Dynamics Fig 6. 22: Dynamics in a model of a C. elegans multi-input FFL following pulses of input stimuli. a) A double input FFL, input functions and thresholds are shown. b) Dynamics of the two-input FFL, with well-separated input pulses For X 1 and X 2, followed by a persistent X 1 stimulus. C) Dynamics With short X 1 stimulus followed rapidly by a short X 2 stimulus. The dashed horizontal line corresponds to the activation thresholds for Y. a=1 was used. (b) Source: Kashtan et al, PRE 2004 70: 031909 (c)

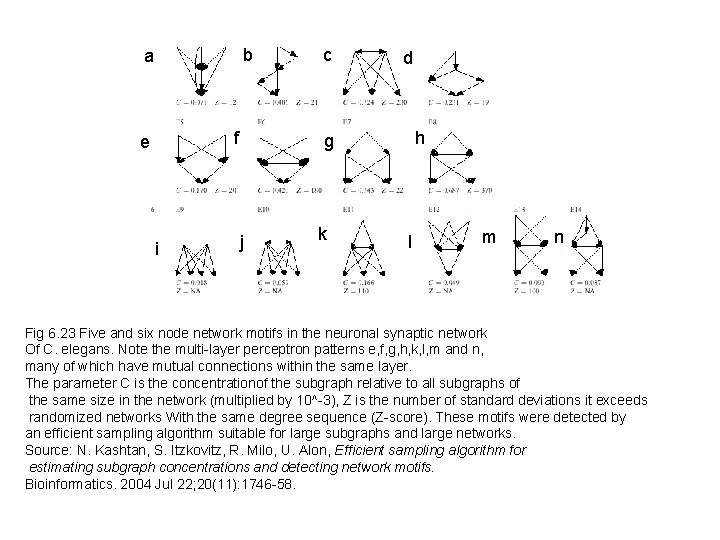
b a f e i c d h g j k l m n Fig 6. 23 Five and six node network motifs in the neuronal synaptic network Of C. elegans. Note the multi-layer perceptron patterns e, f, g, h, k, l, m and n, many of which have mutual connections within the same layer. The parameter C is the concentrationof the subgraph relative to all subgraphs of the same size in the network (multiplied by 10^-3), Z is the number of standard deviations it exceeds randomized networks With the same degree sequence (Z-score). These motifs were detected by an efficient sampling algorithm suitable for large subgraphs and large networks. Source: N. Kashtan, S. Itzkovitz, R. Milo, U. Alon, Efficient sampling algorithm for estimating subgraph concentrations and detecting network motifs. Bioinformatics. 2004 Jul 22; 20(11): 1746 -58.

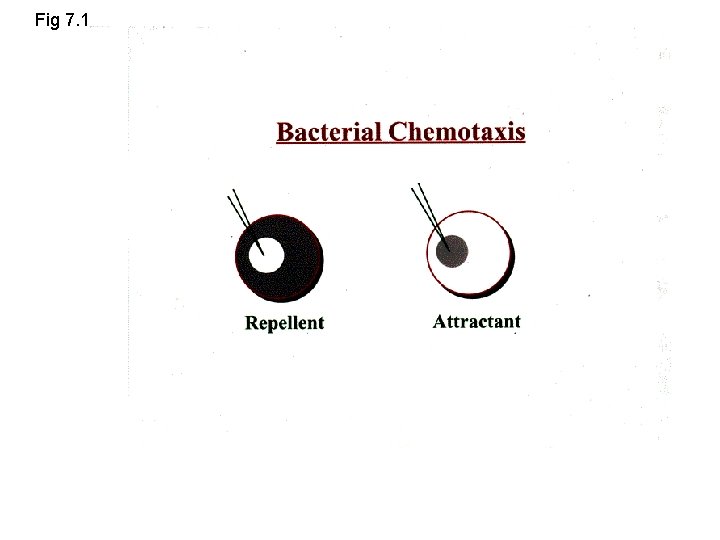
Fig 7. 1

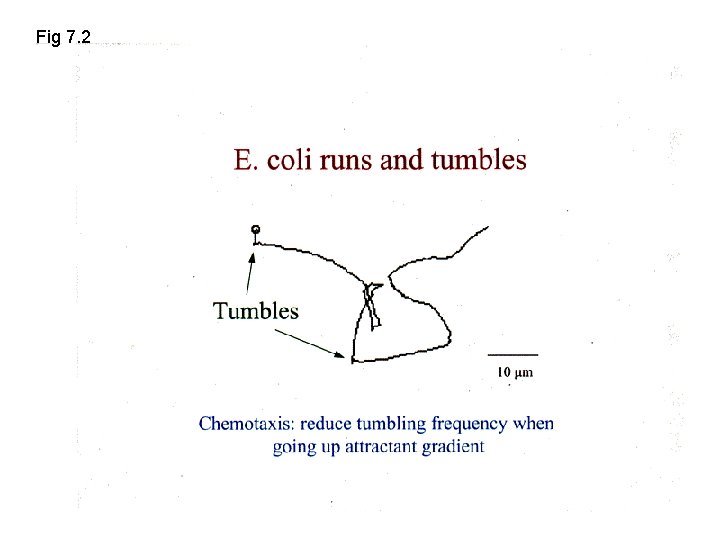
Fig 7. 2
![Fig 7 3 The bacteria flagella motor source Berg HC Ann Rev Biochem 2003 Fig 7. 3 The bacteria flagella motor [source: Berg HC, Ann. Rev. Biochem 2003]](https://slidetodoc.com/presentation_image_h/80cf1540484dd59ba7967c62f6b7d826/image-19.jpg)
Fig 7. 3 The bacteria flagella motor [source: Berg HC, Ann. Rev. Biochem 2003]

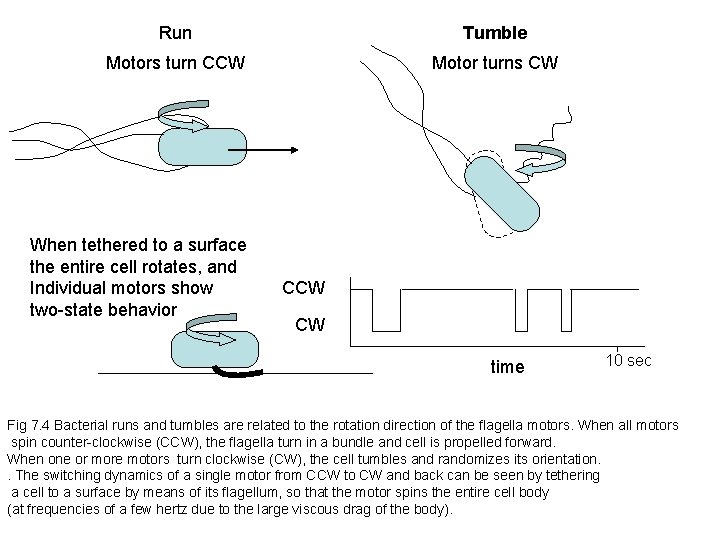
Run Tumble Motors turn CCW Motor turns CW When tethered to a surface the entire cell rotates, and Individual motors show two-state behavior CCW CW time 10 sec Fig 7. 4 Bacterial runs and tumbles are related to the rotation direction of the flagella motors. When all motors spin counter-clockwise (CCW), the flagella turn in a bundle and cell is propelled forward. When one or more motors turn clockwise (CW), the cell tumbles and randomizes its orientation. . The switching dynamics of a single motor from CCW to CW and back can be seen by tethering a cell to a surface by means of its flagellum, so that the motor spins the entire cell body (at frequencies of a few hertz due to the large viscous drag of the body).
![Tumbling frequency 1sec Attractant added exact adaptation Time min Fig 7 5 Average Tumbling frequency 1/sec Attractant added exact adaptation Time [min] Fig 7. 5 : Average](https://slidetodoc.com/presentation_image_h/80cf1540484dd59ba7967c62f6b7d826/image-21.jpg)
Tumbling frequency 1/sec Attractant added exact adaptation Time [min] Fig 7. 5 : Average tumbling-frequency of a population of cells exposed at time t= 5 to a step addition of saturating attractant (such as L-aspartate). After t=5, attractant is uniformly present at constant concentration. Exact adaptation means that the steady-state tumbling-frequency does not depend on the level of attractant.
![Fig 7 6 The chemotaxis signal transduction network source Alon et al Nature 1999 Fig 7. 6 The chemotaxis signal transduction network [source: Alon et al Nature 1999]](https://slidetodoc.com/presentation_image_h/80cf1540484dd59ba7967c62f6b7d826/image-22.jpg)
Fig 7. 6 The chemotaxis signal transduction network [source: Alon et al Nature 1999]

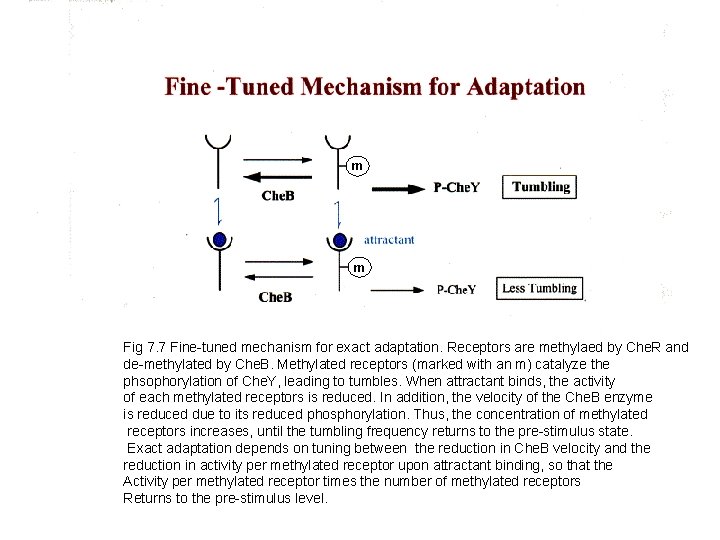
m m Fig 7. 7 Fine-tuned mechanism for exact adaptation. Receptors are methylaed by Che. R and de-methylated by Che. B. Methylated receptors (marked with an m) catalyze the phsophorylation of Che. Y, leading to tumbles. When attractant binds, the activity of each methylated receptors is reduced. In addition, the velocity of the Che. B enzyme is reduced due to its reduced phosphorylation. Thus, the concentration of methylated receptors increases, until the tumbling frequency returns to the pre-stimulus state. Exact adaptation depends on tuning between the reduction in Che. B velocity and the reduction in activity per methylated receptor upon attractant binding, so that the Activity per methylated receptor times the number of methylated receptors Returns to the pre-stimulus level.

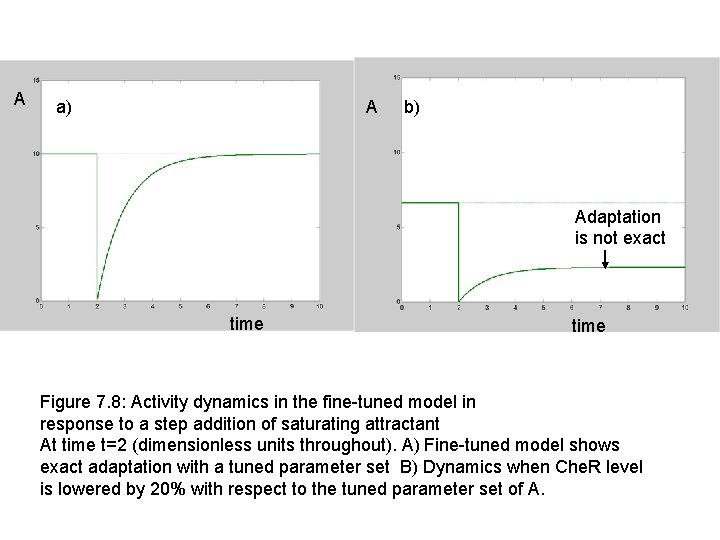
A a) A b) Adaptation is not exact time Figure 7. 8: Activity dynamics in the fine-tuned model in response to a step addition of saturating attractant At time t=2 (dimensionless units throughout). A) Fine-tuned model shows exact adaptation with a tuned parameter set B) Dynamics when Che. R level is lowered by 20% with respect to the tuned parameter set of A.

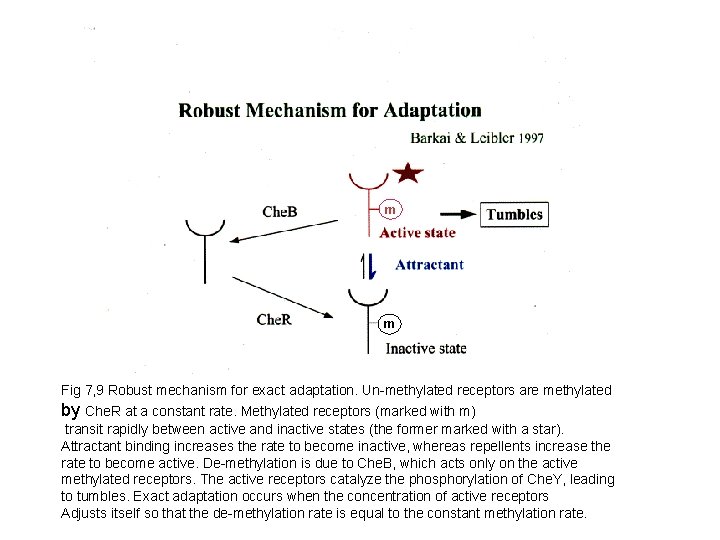
m m Fig 7, 9 Robust mechanism for exact adaptation. Un-methylated receptors are methylated by Che. R at a constant rate. Methylated receptors (marked with m) transit rapidly between active and inactive states (the former marked with a star). Attractant binding increases the rate to become inactive, whereas repellents increase the rate to become active. De-methylation is due to Che. B, which acts only on the active methylated receptors. The active receptors catalyze the phosphorylation of Che. Y, leading to tumbles. Exact adaptation occurs when the concentration of active receptors Adjusts itself so that the de-methylation rate is equal to the constant methylation rate.

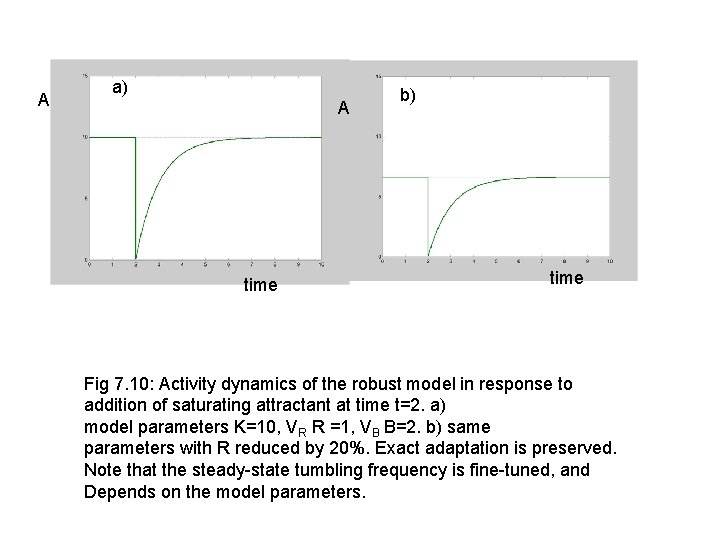
A a) A time b) time Fig 7. 10: Activity dynamics of the robust model in response to addition of saturating attractant at time t=2. a) model parameters K=10, VR R =1, VB B=2. b) same parameters with R reduced by 20%. Exact adaptation is preserved. Note that the steady-state tumbling frequency is fine-tuned, and Depends on the model parameters.

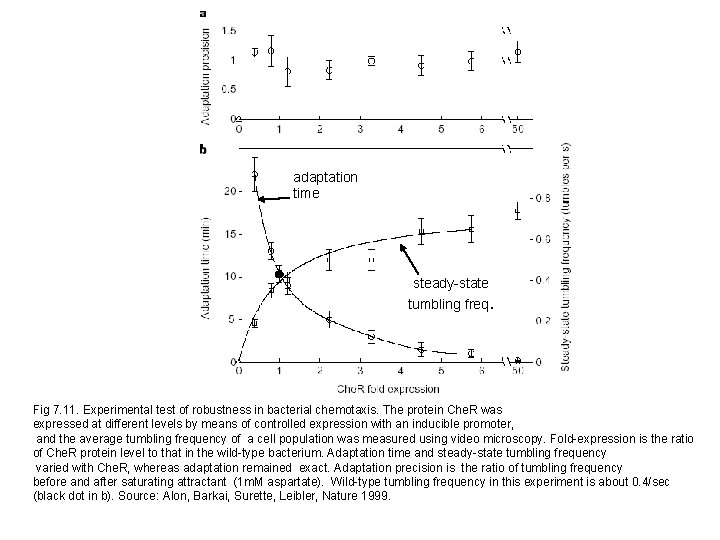
adaptation time steady-state tumbling freq. Fig 7. 11. Experimental test of robustness in bacterial chemotaxis. The protein Che. R was expressed at different levels by means of controlled expression with an inducible promoter, and the average tumbling frequency of a cell population was measured using video microscopy. Fold-expression is the ratio of Che. R protein level to that in the wild-type bacterium. Adaptation time and steady-state tumbling frequency varied with Che. R, whereas adaptation remained exact. Adaptation precision is the ratio of tumbling frequency before and after saturating attractant (1 m. M aspartate). Wild-type tumbling frequency in this experiment is about 0. 4/sec (black dot in b). Source: Alon, Barkai, Surette, Leibler, Nature 1999.

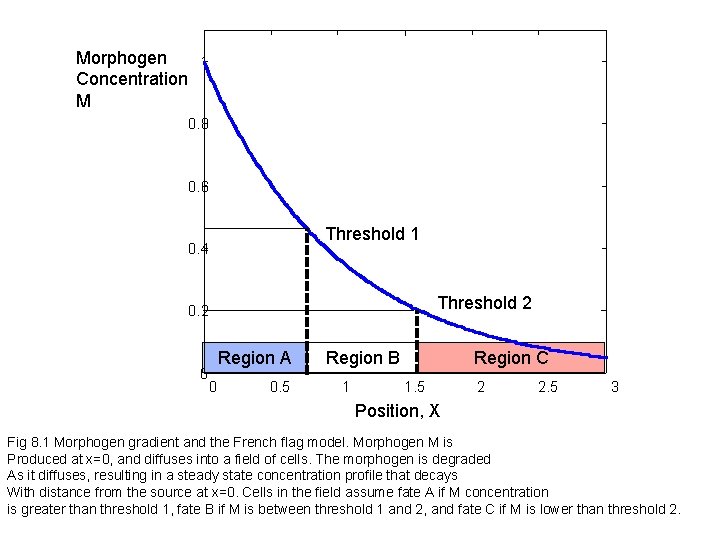
Morphogen 1 Concentration M 0. 8 0. 6 Threshold 1 0. 4 Threshold 2 0 Region AA Region 0 0. 5 Region B B 1 Region C C 1. 5 2 2. 5 3 Position, X Fig 8. 1 Morphogen gradient and the French flag model. Morphogen M is Produced at x=0, and diffuses into a field of cells. The morphogen is degraded As it diffuses, resulting in a steady state concentration profile that decays With distance from the source at x=0. Cells in the field assume fate A if M concentration is greater than threshold 1, fate B if M is between threshold 1 and 2, and fate C if M is lower than threshold 2.

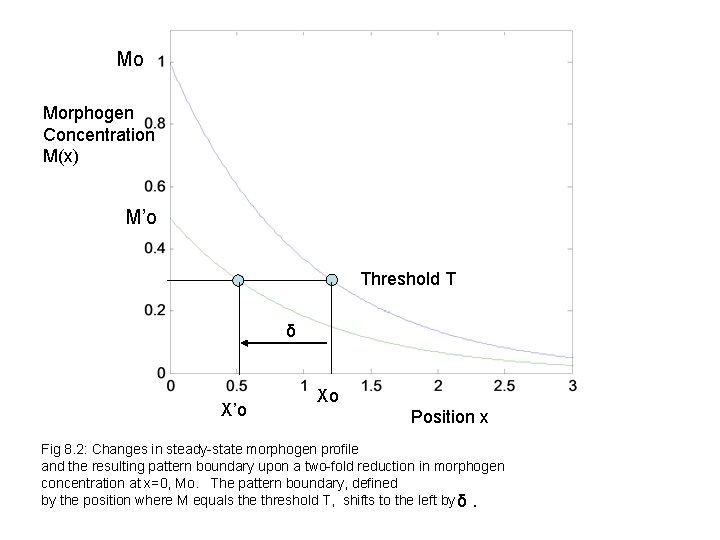
Mo Morphogen Concentration M(x) M’o Threshold T δ X’o Xo Position x Fig 8. 2: Changes in steady-state morphogen profile and the resulting pattern boundary upon a two-fold reduction in morphogen concentration at x=0, Mo. The pattern boundary, defined by the position where M equals the threshold T, shifts to the left by δ.

Mo Morphogen concentration M(x) M’o M(x)=Mo Shift, δ Position x Fig 8. 3, A change in morphogen concentration at th source from Mo to Mo’ leads to a spatially uniform shift in the morphogen profile. All arrows are of equal length. The size of the shift is equal to the position at which M(x)=Mo’.

δ M M δ Fig 8. 4 Comparison of properties of exponential and power-law morphogen profiles. A diffusible morphogen that is subject to linear degradation reaches an exponential profile at steady state (solid line). A perturbed profile (dashed line) was obtained by reducing the morphogen at the boundary, Mo, by a factor e. The resulting shift in cell fate boundary (δ ) is comparable to the distance between two boundaries in the unperturbed profile (Δx). Note the logarithmic scale. (b) When the morphogen undergoes nonlinear self-enhanced degradation, a power-law morphogen profile is established at steady-state. In this case, δ is significantly smaller than Δx. The symbols are the same as in (a), and quadratic degradation was used (Eq 8. 7) Source: Eldar et al Dev Cell. 2003 Oct; 5(4): 635 -46.

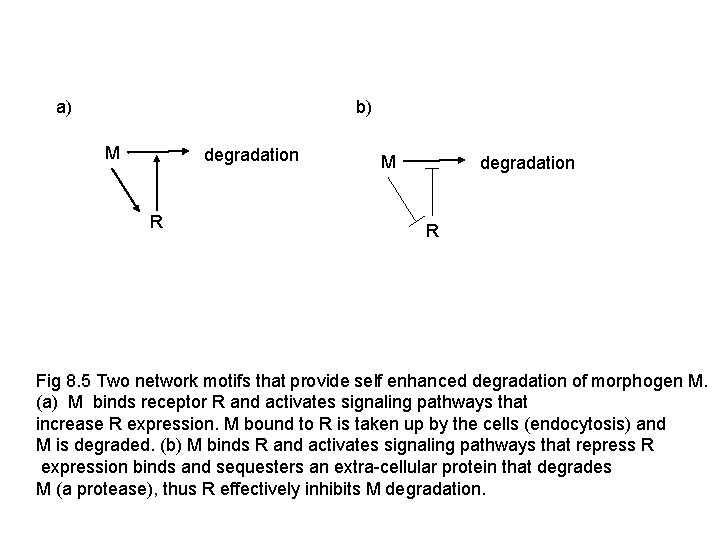
b) a) M degradation R Fig 8. 5 Two network motifs that provide self enhanced degradation of morphogen M. (a) M binds receptor R and activates signaling pathways that increase R expression. M bound to R is taken up by the cells (endocytosis) and M is degraded. (b) M binds R and activates signaling pathways that repress R expression binds and sequesters an extra-cellular protein that degrades M (a protease), thus R effectively inhibits M degradation.

P P--| I --| M M I I DR Fig 8. 6: Cross section of the early drosophila embryo, about 2 h from start of development. Cells are arranged on the periphery of a cylinder. Three cell types are found ( three distinct domains of gene expression) This sets the stage for the patterning considered in this section, in which the dorsal region (DR), which comprises about 50 cells, is to be Sub-patterned. Shown are the regions of expression of the genes of the patterning network: M is the morphogen (Scw, an activating BMP class ligands, also Dpp, expressed in DR); I is an inhibitor of M (Sog); P is a protease (Tld) that cleaves I. Note that M is expressed by all cells, P is expressed only in DR , and expression of I is restricted to the neuroectoderm (NE). (b) Robustness of signaling pathway activity profile in the DR. Pathway activity corresponds to the level of free of M. Robustness Was tested with respect to changes in the gene dosage of M, I and P. Shown are measurement of signaling pathway activity for wild-type cells and mutants with half gene dosage for M (scw+/-) , I (sog+/-)and P(tld+/-), as well as over-expressed P (tld OE). Source: Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. Nature. 2002 Sep 19; 419(6904): 304 -8.

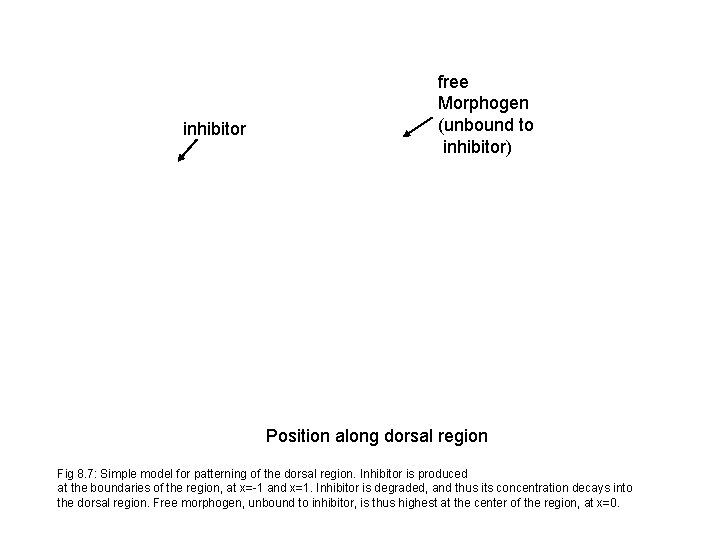
inhibitor free Morphogen (unbound to inhibitor) Position along dorsal region Fig 8. 7: Simple model for patterning of the dorsal region. Inhibitor is produced at the boundaries of the region, at x=-1 and x=1. Inhibitor is degraded, and thus its concentration decays into the dorsal region. Free morphogen, unbound to inhibitor, is thus highest at the center of the region, at x=0.

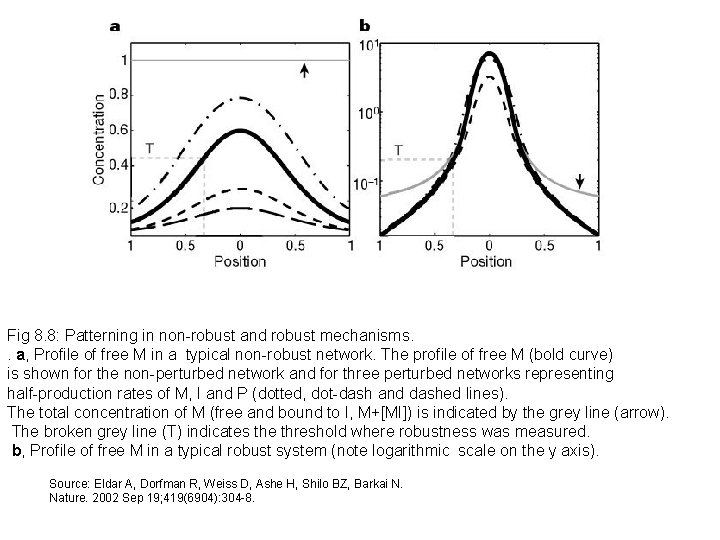
Fig 8. 8: Patterning in non-robust and robust mechanisms. . a, Profile of free M in a typical non-robust network. The profile of free M (bold curve) is shown for the non-perturbed network and for three perturbed networks representing half-production rates of M, I and P (dotted, dot-dash and dashed lines). The total concentration of M (free and bound to I, M+[MI]) is indicated by the grey line (arrow). The broken grey line (T) indicates the threshold where robustness was measured. b, Profile of free M in a typical robust system (note logarithmic scale on the y axis). Source: Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. Nature. 2002 Sep 19; 419(6904): 304 -8.

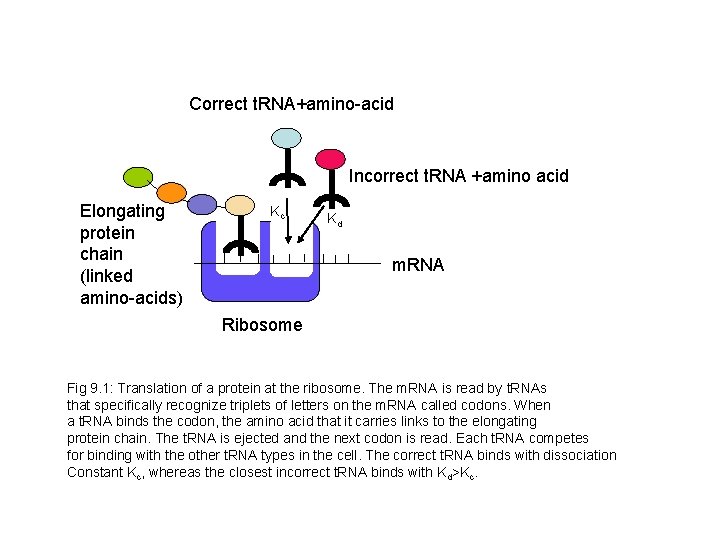
Correct t. RNA+amino-acid Incorrect t. RNA +amino acid Elongating protein chain (linked amino-acids) Kc Kd m. RNA Ribosome Fig 9. 1: Translation of a protein at the ribosome. The m. RNA is read by t. RNAs that specifically recognize triplets of letters on the m. RNA called codons. When a t. RNA binds the codon, the amino acid that it carries links to the elongating protein chain. The t. RNA is ejected and the next codon is read. Each t. RNA competes for binding with the other t. RNA types in the cell. The correct t. RNA binds with dissociation Constant Kc, whereas the closest incorrect t. RNA binds with Kd>Kc.

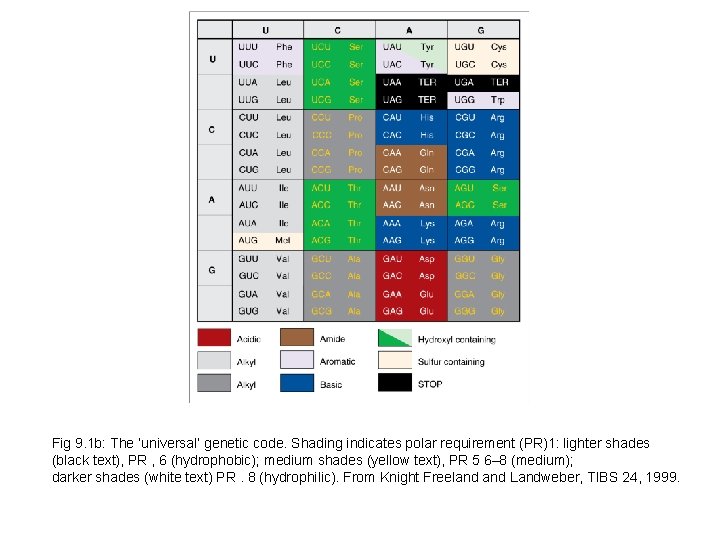
Fig 9. 1 b: The ‘universal’ genetic code. Shading indicates polar requirement (PR)1: lighter shades (black text), PR , 6 (hydrophobic); medium shades (yellow text), PR 5 6– 8 (medium); darker shades (white text) PR. 8 (hydrophilic). From Knight Freeland Landweber, TIBS 24, 1999.

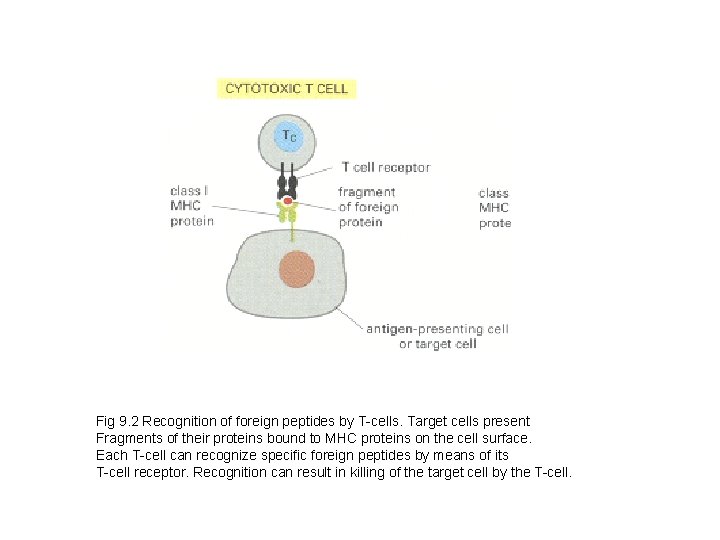
Fig 9. 2 Recognition of foreign peptides by T-cells. Target cells present Fragments of their proteins bound to MHC proteins on the cell surface. Each T-cell can recognize specific foreign peptides by means of its T-cell receptor. Recognition can result in killing of the target cell by the T-cell.

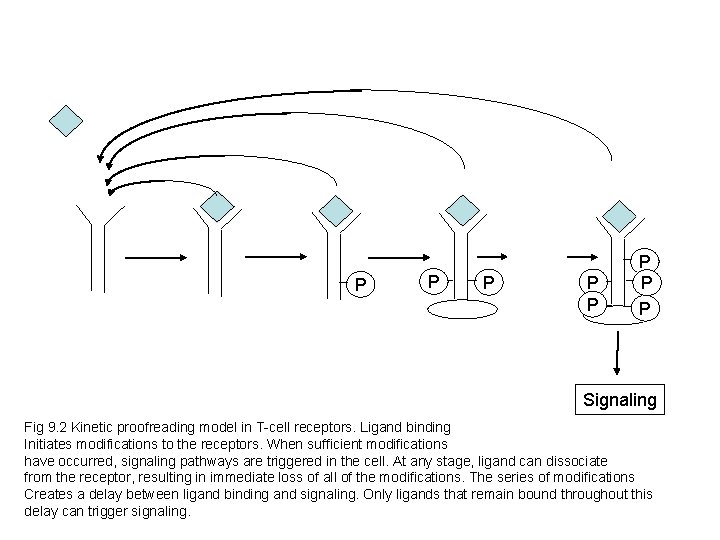
P P P P Signaling Fig 9. 2 Kinetic proofreading model in T-cell receptors. Ligand binding Initiates modifications to the receptors. When sufficient modifications have occurred, signaling pathways are triggered in the cell. At any stage, ligand can dissociate from the receptor, resulting in immediate loss of all of the modifications. The series of modifications Creates a delay between ligand binding and signaling. Only ligands that remain bound throughout this delay can trigger signaling.

Optimal gene circuit design

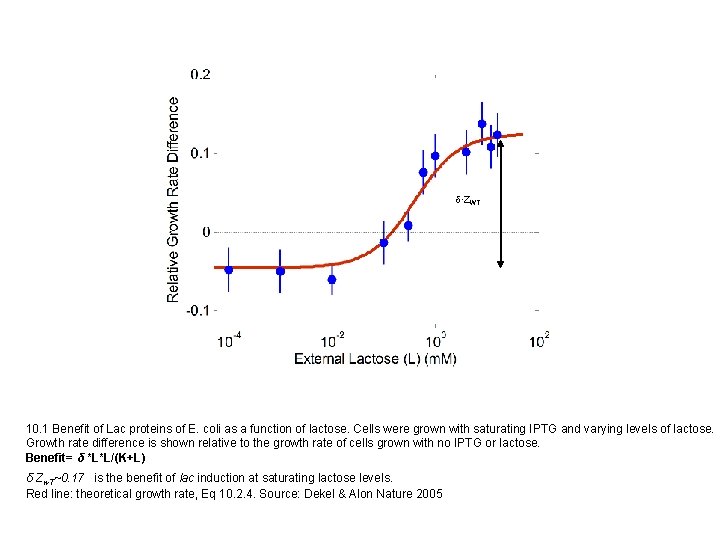
d∙ZWT 10. 1 Benefit of Lac proteins of E. coli as a function of lactose. Cells were grown with saturating IPTG and varying levels of lactose. Growth rate difference is shown relative to the growth rate of cells grown with no IPTG or lactose. Benefit= δ *L*L/(K+L) δ Zw. T~0. 17 is the benefit of lac induction at saturating lactose levels. Red line: theoretical growth rate, Eq 10. 2. 4. Source: Dekel & Alon Nature 2005

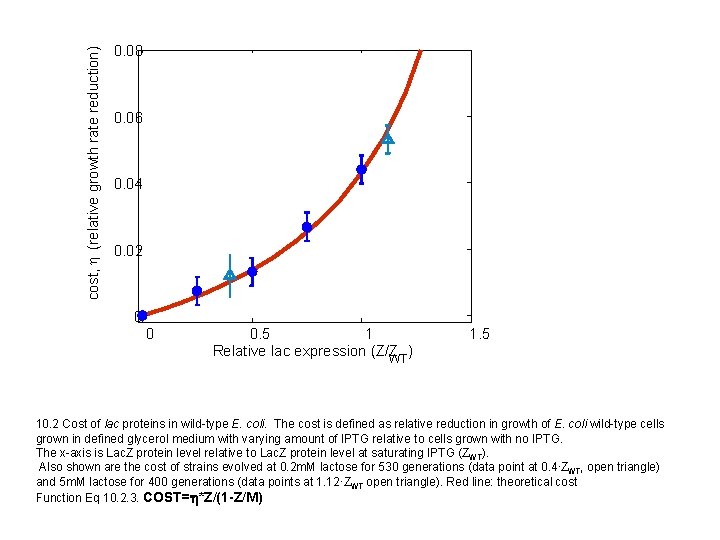
cost, h (relative growth rate reduction) 0. 08 0. 06 0. 04 0. 02 0 0 0. 5 1 Relative lac expression (Z/Z ) WT 1. 5 10. 2 Cost of lac proteins in wild-type E. coli. The cost is defined as relative reduction in growth of E. coli wild-type cells grown in defined glycerol medium with varying amount of IPTG relative to cells grown with no IPTG. The x-axis is Lac. Z protein level relative to Lac. Z protein level at saturating IPTG (ZWT). Also shown are the cost of strains evolved at 0. 2 m. M lactose for 530 generations (data point at 0. 4∙ZWT, open triangle) and 5 m. M lactose for 400 generations (data points at 1. 12·ZWT open triangle). Red line: theoretical cost Function Eq 10. 2. 3. COST=h*Z/(1 -Z/M)

10. 3 Predicted relative growth rate of cells (the 'fitness function') as a function of Lac protein expression, in different concentrations of lactose, based on the experimentally measured cost and benefit functions. The x-axis is the ratio of protein level to the fully induced wild-type protein level, Z/ZWT. Shown are relative growth differences with respect to the un-induced wild-type strain, for environments with lactose levels L=0. 1 m. M (blue line), L=0. 6 m. M (green line) and L=5 m. M (red Line), according to Eq (5). The dot on each line is the predicted optimal expression level which provides maximal growth, Eq 6. Cells grown in lactose levels above 0. 6 m. M are predicted to evolve to increased Lac protein expression (top arrow ), whereas cells grown at lactose levels lower than 0. 6 m. M are predicted to evolve to decreased expression (lower arrow ).

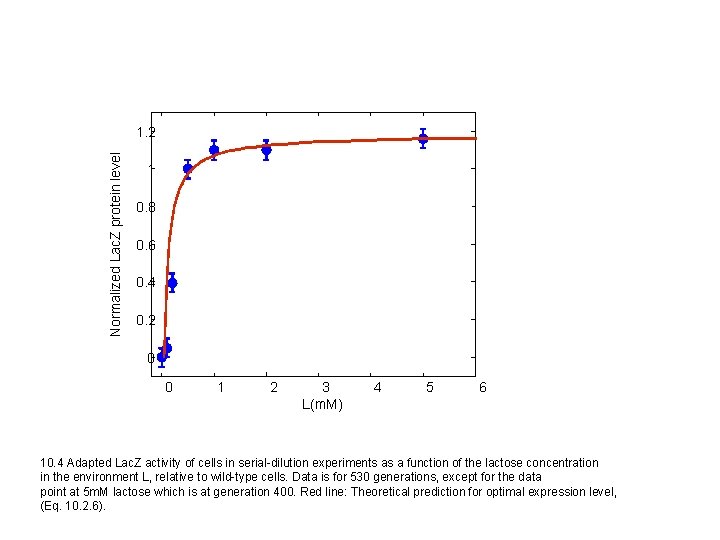
Normalized Lac. Z protein level 1. 2 1 0. 8 0. 6 0. 4 0. 2 0 0 1 2 3 L(m. M) 4 5 6 10. 4 Adapted Lac. Z activity of cells in serial-dilution experiments as a function of the lactose concentration in the environment L, relative to wild-type cells. Data is for 530 generations, except for the data point at 5 m. M lactose which is at generation 400. Red line: Theoretical prediction for optimal expression level, (Eq. 10. 2. 6).

Lac. Z Protein Level 1. 2 5 m. M Lactose 2 m. M Lactose 1 0. 5 m. M Lactose 0. 8 0. 6 0. 1 m. M Lactose No Lactose 0. 4 0. 2 0 100 200 300 400 Generations 500 : 10. 5 Experimental evolutionary adaptation of E. coli cells to different concentrations of lactose. Lac. Z activity relative to wild-type cells of cells grown for 530 generations in serial dilution experiments with different lactose levels is shown as a function of generation number. Cells were grown in 0, 0. 1 m. M, 0. 5 m. M, 2 m. M and 5 m. M lactose in a glycerol minimal medium supplemented with 0. 15 m. M IPTG. Lines are population genetics simulations of the serial dilution conditions. The only fitting parameter in these simulations is the probability per cell division for a mutation that yields the adapted lac. Z level.

To regulate or Not regulate:

Variable environment • Suppose Z is produced with benefit only in the environment condition Cz with prob. p • Three strategy: 1. Unregulated f 1=p*b-c 2. Regulated f 2=p*b-c*p-r 3. Lack of gene Z f 3=0 no benefit b no cost c

Variable environment • Regulation wins: f 2> f 1 and f 2>f 3 p<1 -r/c and p > r/(b-c) • Non regulation wins: p > c/b and p > 1 - r/c

Variable environment • If p=1 always Cz Z should be always expressed (f 1 best) • If p=0 Cz never happens and Z should not be expressed (f 3 best) • If the environment is sufficiently variables f 2 is the best

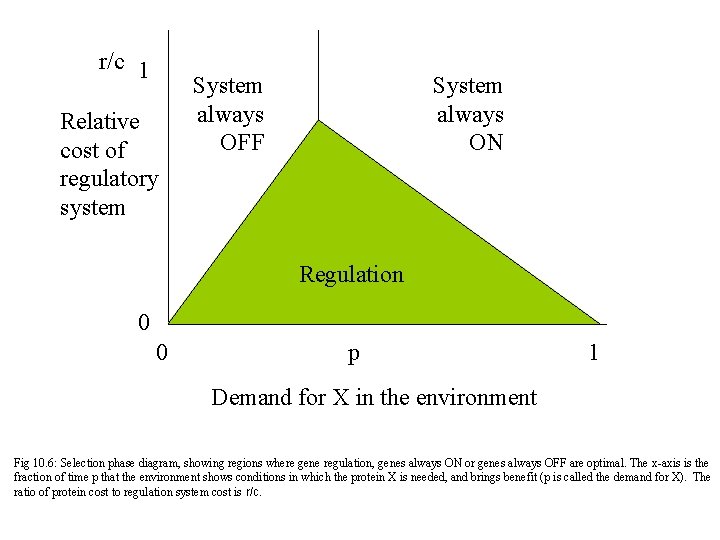
r/c 1 Relative cost of regulatory system System always OFF System always ON Regulation 0 0 p 1 Demand for X in the environment Fig 10. 6: Selection phase diagram, showing regions where gene regulation, genes always ON or genes always OFF are optimal. The x-axis is the fraction of time p that the environment shows conditions in which the protein X is needed, and brings benefit (p is called the demand for X). The ratio of protein cost to regulation system cost is r/c.

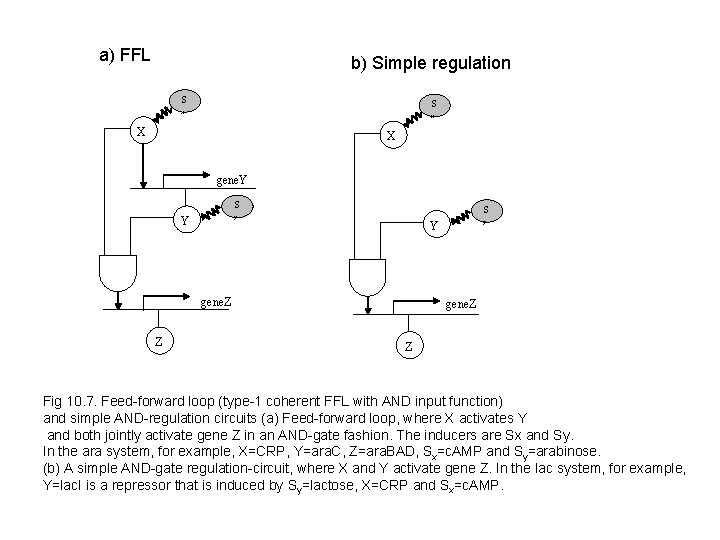
a) FFL b) Simple regulation S S x x X X gene. Y S S y Y gene. Z Z Fig 10. 7. Feed-forward loop (type-1 coherent FFL with AND input function) and simple AND-regulation circuits (a) Feed-forward loop, where X activates Y and both jointly activate gene Z in an AND-gate fashion. The inducers are Sx and Sy. In the ara system, for example, X=CRP, Y=ara. C, Z=ara. BAD, Sx=c. AMP and Sy=arabinose. (b) A simple AND-gate regulation-circuit, where X and Y activate gene Z. In the lac system, for example, Y=lac. I is a repressor that is induced by Sy=lactose, X=CRP and Sx=c. AMP.

Growth rate integrated over Input pulse duration (D) Fig 10. 8: Integrated fitness (integrated growth rate) of simple regulation during short pulses of inputs Sx and Sy. Fitness is negative for D<Dc.

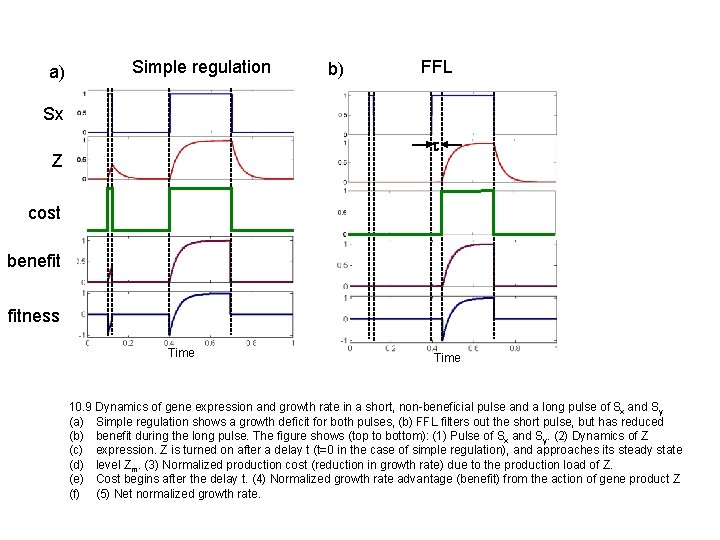
a) Simple regulation b) FFL Sx t Z cost benefit fitness Time 10. 9 Dynamics of gene expression and growth rate in a short, non-beneficial pulse and a long pulse of Sx and Sy (a) Simple regulation shows a growth deficit for both pulses, (b) FFL filters out the short pulse, but has reduced (b) benefit during the long pulse. The figure shows (top to bottom): (1) Pulse of Sx and Sy. (2) Dynamics of Z (c) expression. Z is turned on after a delay t (t=0 in the case of simple regulation), and approaches its steady state (d) level Zm. (3) Normalized production cost (reduction in growth rate) due to the production load of Z. (e) Cost begins after the delay t. (4) Normalized growth rate advantage (benefit) from the action of gene product Z (f) (5) Net normalized growth rate.

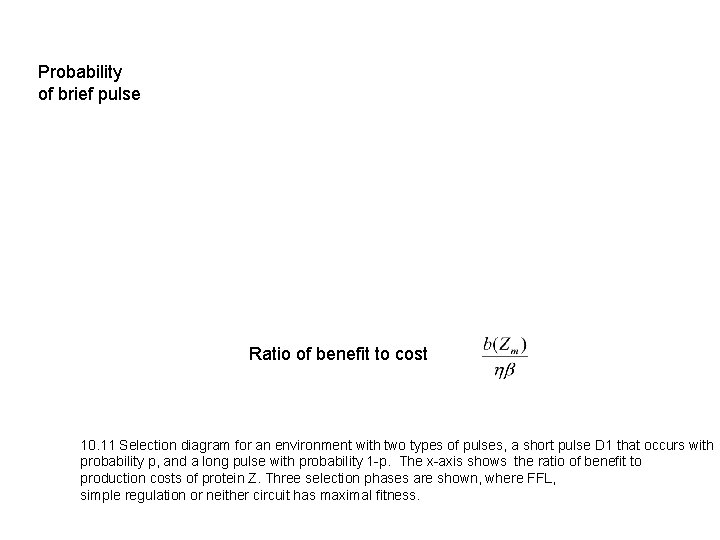
Probability of brief pulse Ratio of benefit to cost 10. 11 Selection diagram for an environment with two types of pulses, a short pulse D 1 that occurs with probability p, and a long pulse with probability 1 -p. The x-axis shows the ratio of benefit to production costs of protein Z. Three selection phases are shown, where FFL, simple regulation or neither circuit has maximal fitness.

FFL can be selected in a environment that have deleterious short pulses

Why some genes are regulated by repressors and others by activators?

Positive control (activator) Negative control (repressor) Fig 11. 1: Internal-representations in transcription regulation of an inducible gene Z. The gene is expressed at high level (Z=Z 1), when the input stimulus is present (X=1), and at low level (Z=Z 0) when the stimulus is absent (X=0). This input-output relation can be implemented by two internal-representations, which correspond to positive or negative control. (a) Positive control. Transcription activator protein Y can be either bound (Y=1) or unbound (Y=0) to the promoter of gene Z. When Y is bound, the transcription rate of gene Z is increased. (b) Internal-representation in the case of positive control. The internal-states have errors in which Y can mis-bind to or mis-release from the promoter. (c) Negative control. Repressor protein Y can be either bou expression (Z=Z 0), or unbound (Y=0), resulting in high expression (Z=Z 1). (d) Internal-representation in the case of negative control. Mis-binding and mis-release error standard deviations are σ0 and σ1.

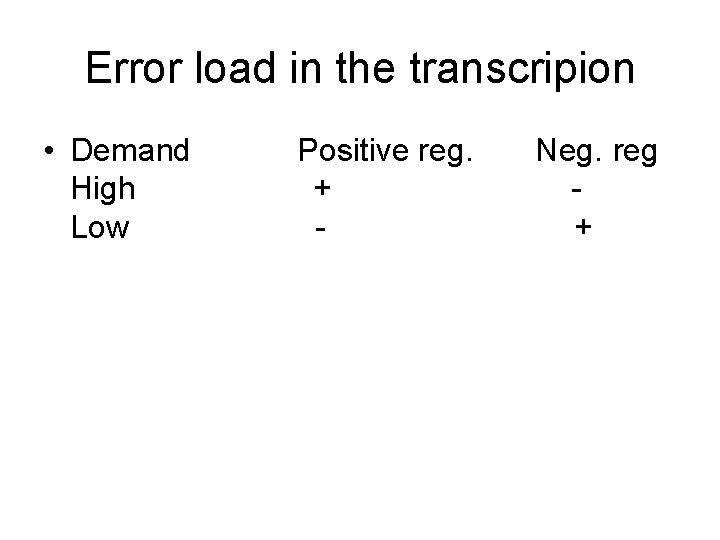
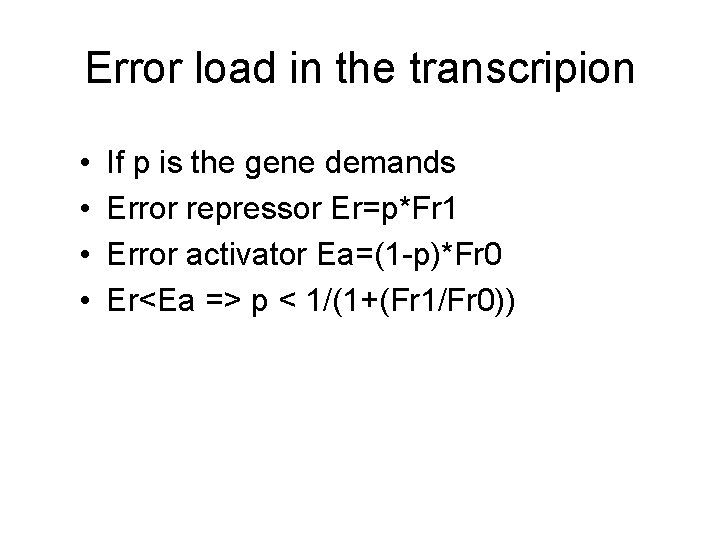
Error load in the transcripion • Demand High Low Positive reg. + - Neg. reg +

Error load in the transcripion • • If p is the gene demands Error repressor Er=p*Fr 1 Error activator Ea=(1 -p)*Fr 0 Er<Ea => p < 1/(1+(Fr 1/Fr 0))

Fig 11. 2 Mis-binding and mis-release error variances arising from cell-cell variability in the concentration of a simple transcription regulator such as CRP. Transcription regulator protein Y can be in an active state (Y=1) in which it has a high affinity to its DNA site, or in an inactive state (Y=0) in which it has a low affinity to the site. In the case of CRP, Y=1 corresponds to CRP bound to The small signaling molecule c. AMP, and Y=0 to CRP not bound to c. AMP. The binding probability of the DNA site iin the promoter i given by the corresponding binding curves. The concentration of CRP and many other bacterial regulators is of the same order of magnitude as its dissociation constant in the inactive state, and thus corresponds to a relatively steep part of the binding curve. Cell-cell variability in the concentration of protein Y leads to a large standard deviation around the mean binding probability at state Y=0, and to a smaller standard deviation at state Y=1. The mis-binding error standard deviation, σ0 is thus smaller than the mis-release error standard deviation, σ1. Note that for CRP, the concentration of Y in the active and in active states is about the same, which is approximately true also for many other bacterial regulators.

Fig 11. 3 Demand Fig 11. 3: Selection diagram for the mode of regulation of a gene by a single regulator, as a function of the environmental demand p for the gene and the ratio of the costs C 1/C 0. Positive control corresponds to a transcription activator, and negative control to a repressor. This selection diagram applies to the case Where mis binding variance exceeds mis-release variance.

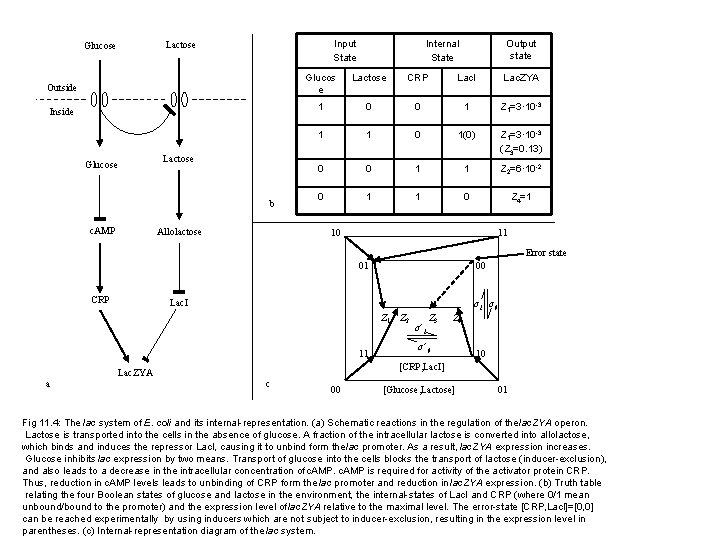
Glucose e Input State Lactose Outside Inside Glucose e Lactose b c. AMP Allolactose Internal State Output state Glucos e Lactose CRP Lac. I Lac. ZYA 1 0 0 1 Z 1=3· 10 -3 1 1 0 1(0) Z 1=3· 10 -3 (Z 3=0. 13) 0 0 1 1 Z 2=6· 10 -2 0 1 1 0 Z 4=1 10 11 Error state 01 CRP 00 Lac. I σ1 σ0 Z 1 11 a Z 2 σ’ 1 Z 3 Z 4 σ’ 0 10 [CRP, Lac. I] Lac. ZYA c 00 [Glucose, Lactose] 01 Fig 11. 4: The lac system of E. coli and its internal-representation. (a) Schematic reactions in the regulation of the lac. ZYA operon. Lactose is transported into the cells in the absence of glucose. A fraction of the intracellular lactose is converted into allolactose, which binds and induces the repressor Lac. I, causing it to unbind form the lac promoter. As a result, lac. ZYA expression increases. Glucose inhibits lac expression by two means. Transport of glucose into the cells blocks the transport of lactose (inducer-exclusion), and also leads to a decrease in the intracellular concentration of c. AMP is required for activity of the activator protein CRP. Thus, reduction in c. AMP levels leads to unbinding of CRP form the lac promoter and reduction in lac. ZYA expression. (b) Truth table relating the four Boolean states of glucose and lactose in the environment, the internal-states of Lac. I and CRP (where 0/1 mean unbound/bound to the promoter) and the expression level of lac. ZYA relative to the maximal level. The error-state [CRP, Lac. I]=[0, 0] can be reached experimentally by using inducers which are not subject to inducer-exclusion, resulting in the expression level in parentheses. (c) Internal-representation diagram of the lac system.

AR AA 10 11 01 11 00 00 Z 1 Z 2 Z 3 Z 4 11 00 10 01 Z 2 10 [Glucose, Lactose] Z 4 10 01 a 00 11 [Glucose, Lactose] 01 c RR RA 10 11 11 10 Z 1 Z 2 Z 3 10 Z 4 01 00 b Z 3 11 Z 2 Z 4 00 00 [Glucose, Lactose] Z 3 01 00 01 [Glucose, Lactose] 01 d Fig 11. 5 The four equivalent internal-representations for the lac system. The internal-representations are labeled by the mode of regulation of the glucose and the lactose responsive regulators, where A/R means activator/repressor. Note that all equivalent internal-representations map the input states on the output states in the same way, and differ only in the internal-states used to realize this mapping.

Fig 11. 6 Selection diagram for the lac system, indicating the internal-representation that minimizes the error-load at each environmental state p 00, p 01. The axes are the probability for neither glucose nor lactose in the environment, p 00, and for lactose in the absence of glucose, p 01. Three of the internal-representations shown in Fig 11. 5 minimize the error-load. The wild-type internal-representation, [AR], minimizes the error-load in a region that includes environments where both sugars are rare. Also shown are the mathematical expressions for the region boundaries.

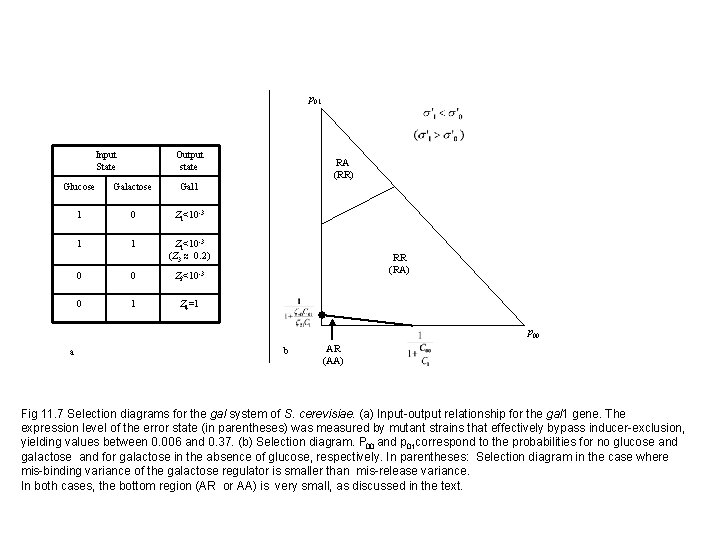
p 01 Input State Output state Glucose Galactose Gal 1 1 0 Z 1<10 -3 1 1 Z 1<10 -3 (Z 3 ≈ 0. 2) 0 0 Z 2<10 -3 0 1 Z 4=1 RA (RR) RR (RA) p 00 a b AR (AA) Fig 11. 7 Selection diagrams for the gal system of S. cerevisiae. (a) Input-output relationship for the gal 1 gene. The expression level of the error state (in parentheses) was measured by mutant strains that effectively bypass inducer-exclusion, yielding values between 0. 006 and 0. 37. (b) Selection diagram. P 00 and p 01 correspond to the probabilities for no glucose and galactose and for galactose in the absence of glucose, respectively. In parentheses: Selection diagram in the case where mis-binding variance of the galactose regulator is smaller than mis-release variance. In both cases, the bottom region (AR or AA) is very small, as discussed in the text.

goal 1 goal 2 12. 1 Electronic circuits evolved under a fixed goal and modularly varying goals. Networks made of logic gates (NAND gates that compute a NOT-AND function on two inputs) were evolved to attain a goal, defined as a logic function of four inputs X, Y, Z and Z. a. A typical circuit evolved by fixed goal evolution towards goal G 1=(X XOR Y) AND (Z XOR W), where XOR is the exclusive OR function. Similar non-modular solutions were found for goal G 2=)X XOR Y( OR )Z XOR W( b. Circuits evolved with modularly varying goals, in which the goal switched between G 1 and G 2 every 20 generations. Note that G 1 and G 2 share the same subproblems (namely X XOR Y, Z XOR W ), but in different combinations. Connections that are rewired when the goal is switched are marked in red. c. Another example of a circuit evolved with modularly varying goals evolution. The circuit shows feedbacks between two of the lower three NAND gates. d. Modular structure of the circuits evolved under modularly varying goals: The circuits are composed of two XOR modules which input into a third module that implements an AND/OR function, depending on the goal. For more details, see Kashtan & Alon, PNAS 2005.

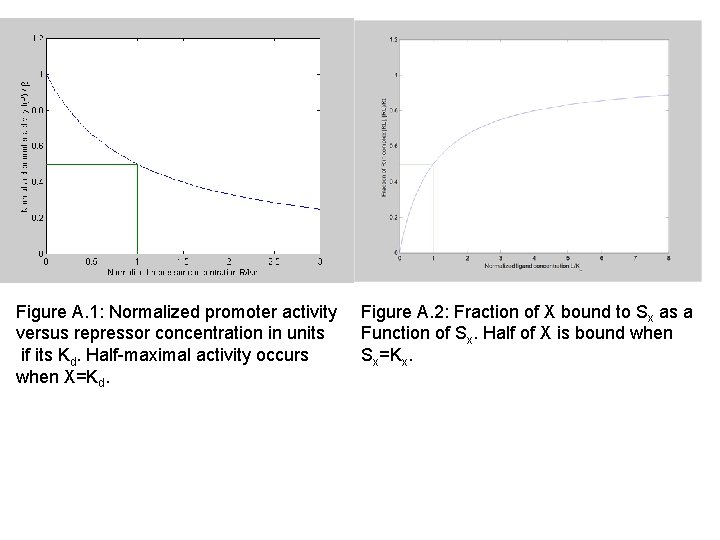
Figure A. 1: Normalized promoter activity versus repressor concentration in units if its Kd. Half-maximal activity occurs when X=Kd. Figure A. 2: Fraction of X bound to Sx as a Function of Sx. Half of X is bound when Sx=Kx.

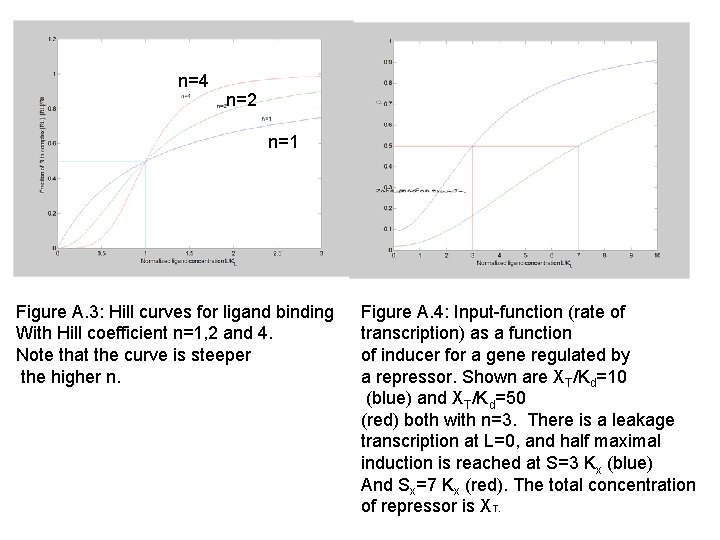
n=4 n=2 n=1 Figure A. 3: Hill curves for ligand binding With Hill coefficient n=1, 2 and 4. Note that the curve is steeper the higher n. Figure A. 4: Input-function (rate of transcription) as a function of inducer for a gene regulated by a repressor. Shown are XT/Kd=10 (blue) and XT/Kd=50 (red) both with n=3. There is a leakage transcription at L=0, and half maximal induction is reached at S=3 Kx (blue) And Sx=7 Kx (red). The total concentration of repressor is XT.

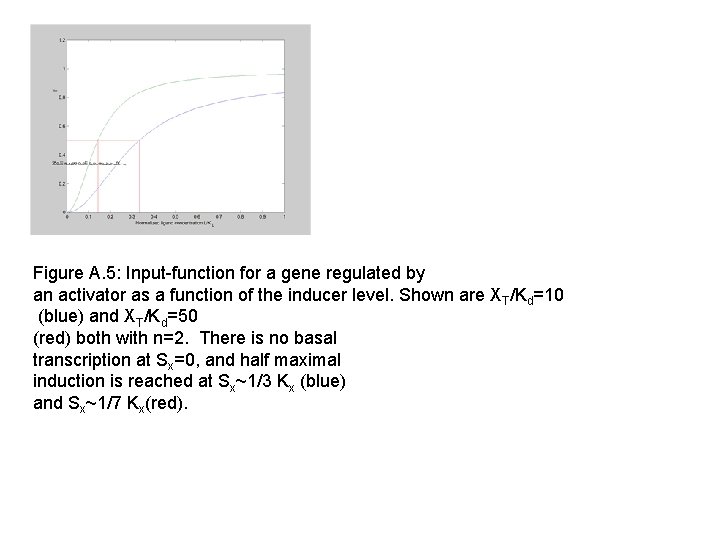
Figure A. 5: Input-function for a gene regulated by an activator as a function of the inducer level. Shown are XT/Kd=10 (blue) and XT/Kd=50 (red) both with n=2. There is no basal transcription at Sx=0, and half maximal induction is reached at Sx~1/3 Kx (blue) and Sx~1/7 Kx(red).

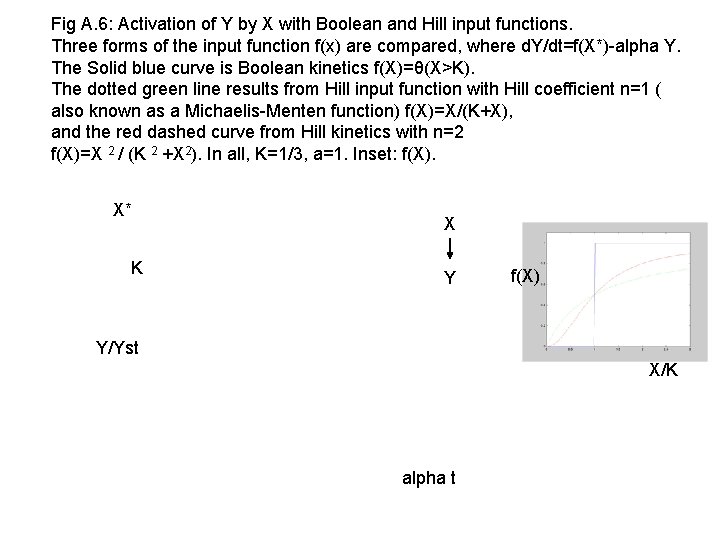
Fig A. 6: Activation of Y by X with Boolean and Hill input functions. Three forms of the input function f(x) are compared, where d. Y/dt=f(X*)-alpha Y. The Solid blue curve is Boolean kinetics f(X)=θ(X>K). The dotted green line results from Hill input function with Hill coefficient n=1 ( also known as a Michaelis-Menten function) f(X)=X/(K+X), and the red dashed curve from Hill kinetics with n=2 f(X)=X 2 / (K 2 +X 2). In all, K=1/3, a=1. Inset: f(X). X* K X Y f(X) Y/Yst X/K alpha t

(a) Full repression by Y (b) Partial repression by Y Fig B. 1 INPUT FUNCTION OF GENE REGULATED BY ACTIVATOR X AND REPRESSOR Y Input-function (expression of Z regulated by activator X and repressor Y) with βz = 1 and (a) full repression β' z =0, and (b) With partial repression β'z=0. 3 (b). In both cases, K 1=K 2=10.