x Energy Analysis of Underground Coal Gasification with

- Slides: 20

x Energy Analysis of Underground Coal Gasification with Simultaneous Storage of Carbon Dioxide Ali Akbar Eftekhari Hans Bruining

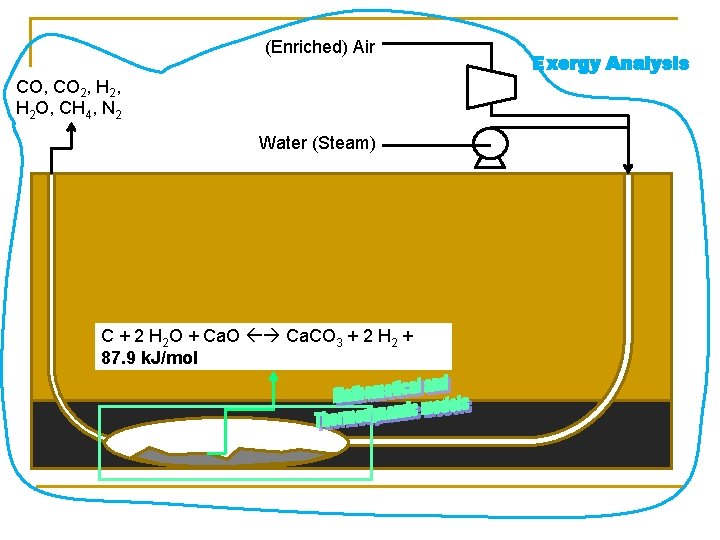
(Enriched) Air CO, CO 2, H 2 O, CH 4, N 2 Water (Steam) C + 2 H 2 O + Ca. O Ca. CO 3 + 2 H 2 + 87. 9 k. J/mol Exergy Analysis

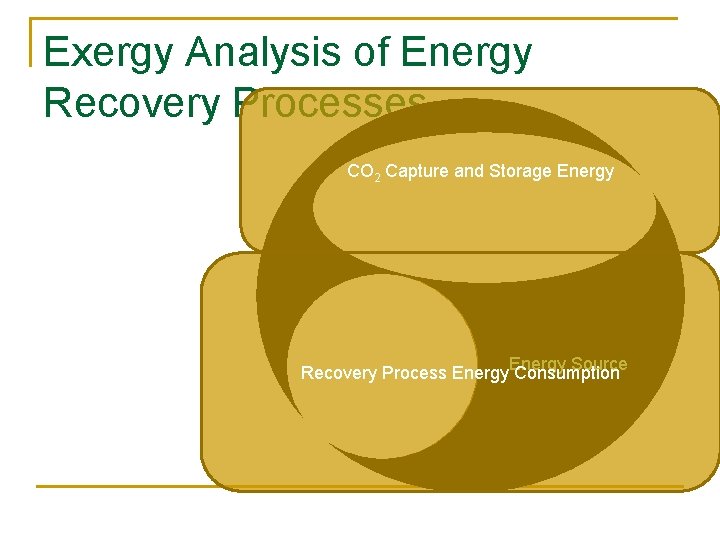
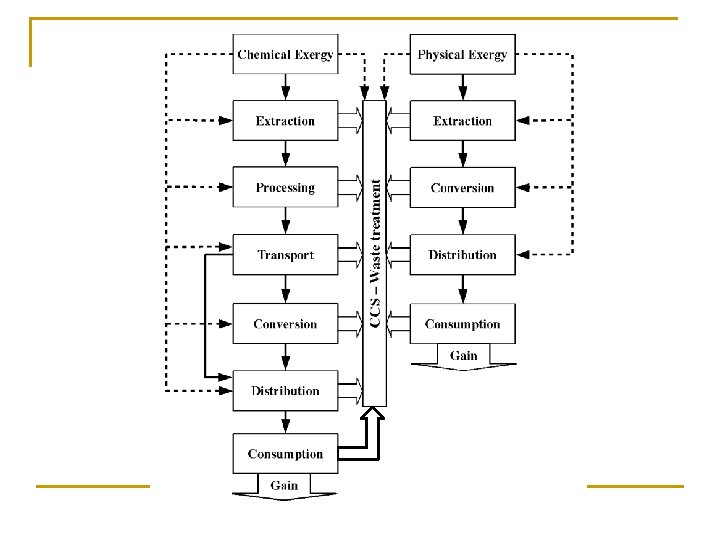
Exergy Analysis of Energy Recovery Processes CO 2 Capture and Storage Energy CO 2 Capture and Storage Recovery Process Source Recovery Process Energy Consumption

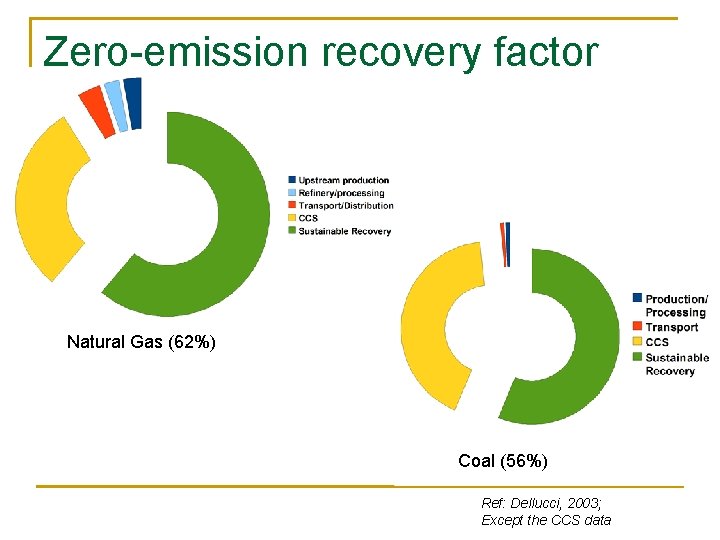
Zero-emission recovery factor Natural Gas (62%) Coal (56%) Ref: Dellucci, 2003; Except the CCS data

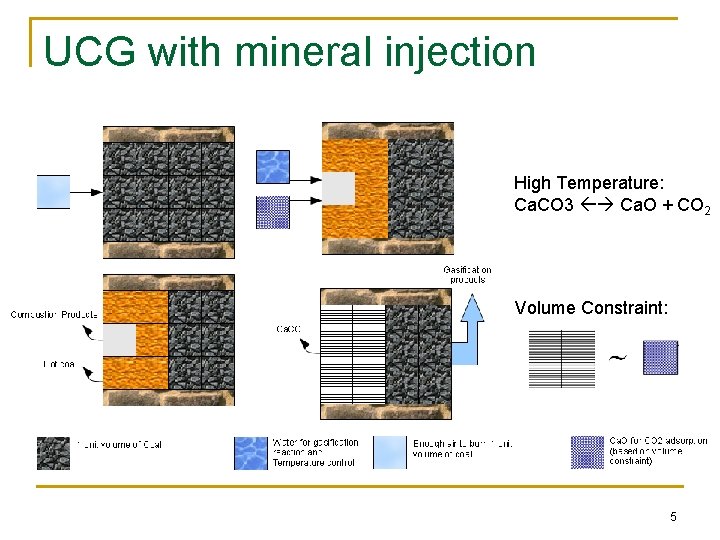
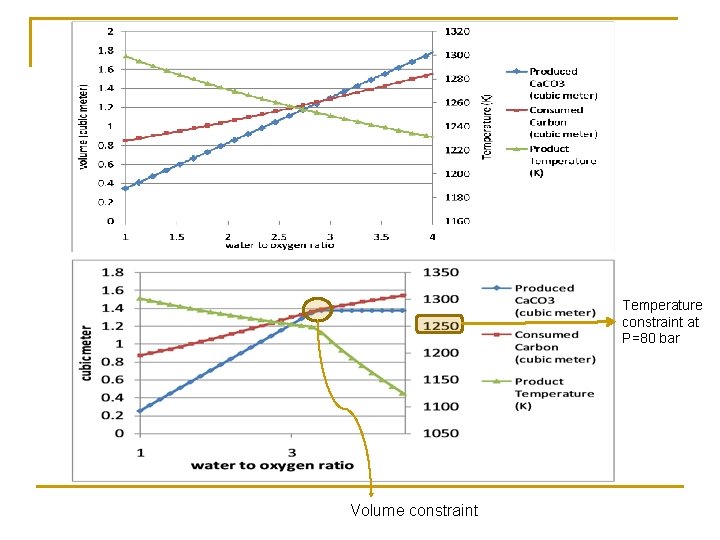
UCG with mineral injection High Temperature: Ca. CO 3 Ca. O + CO 2 Volume Constraint: 5

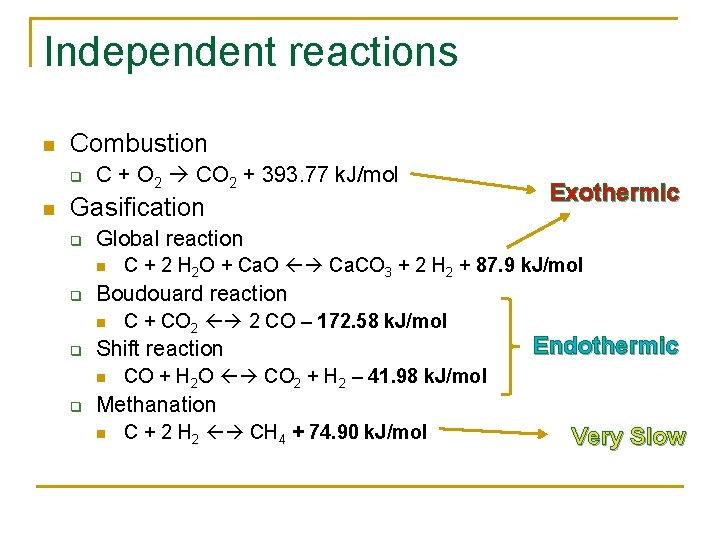
Independent reactions n Combustion q n C + O 2 CO 2 + 393. 77 k. J/mol Gasification q Global reaction n q C + CO 2 2 CO – 172. 58 k. J/mol Shift reaction n q C + 2 H 2 O + Ca. O Ca. CO 3 + 2 H 2 + 87. 9 k. J/mol Boudouard reaction n q Exothermic Endothermic CO + H 2 O CO 2 + H 2 – 41. 98 k. J/mol Methanation n C + 2 H 2 CH 4 + 74. 90 k. J/mol Very Slow

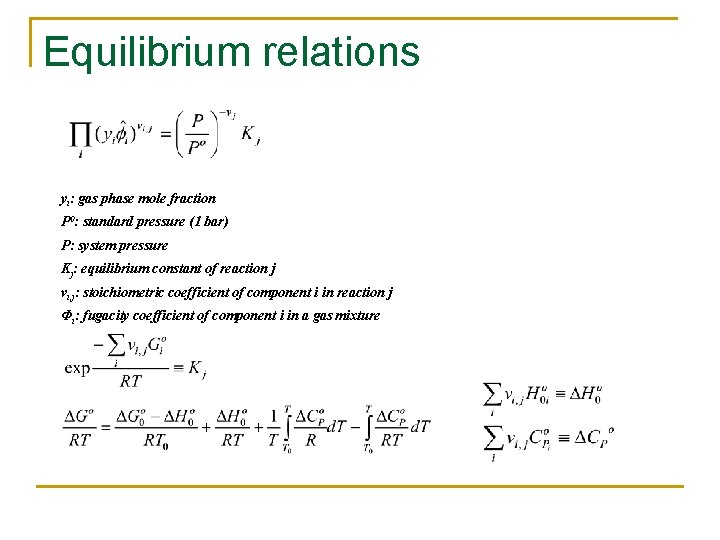
Equilibrium relations yi: gas phase mole fraction P 0: standard pressure (1 bar) P: system pressure Kj: equilibrium constant of reaction j vi, j: stoichiometric coefficient of component i in reaction j Φi: fugacity coefficient of component i in a gas mixture

Temperature constraint at P=80 bar Volume constraint

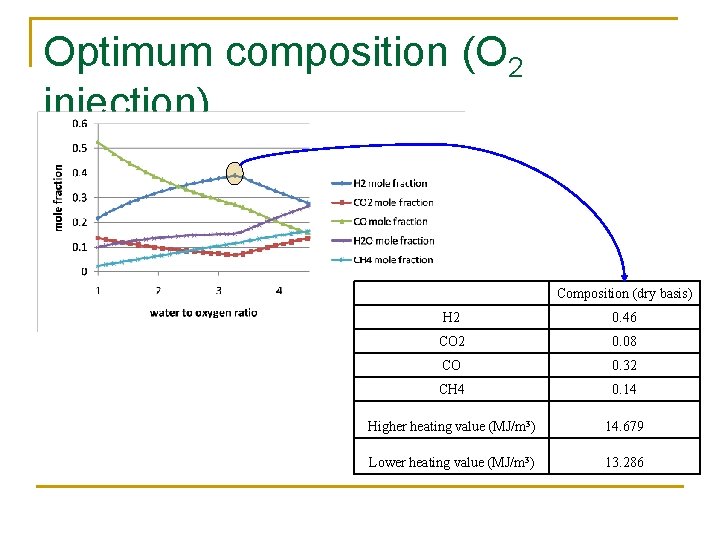
Optimum composition (O 2 injection) Composition (dry basis) H 2 0. 46 CO 2 0. 08 CO 0. 32 CH 4 0. 14 Higher heating value (MJ/m 3) 14. 679 Lower heating value (MJ/m 3) 13. 286

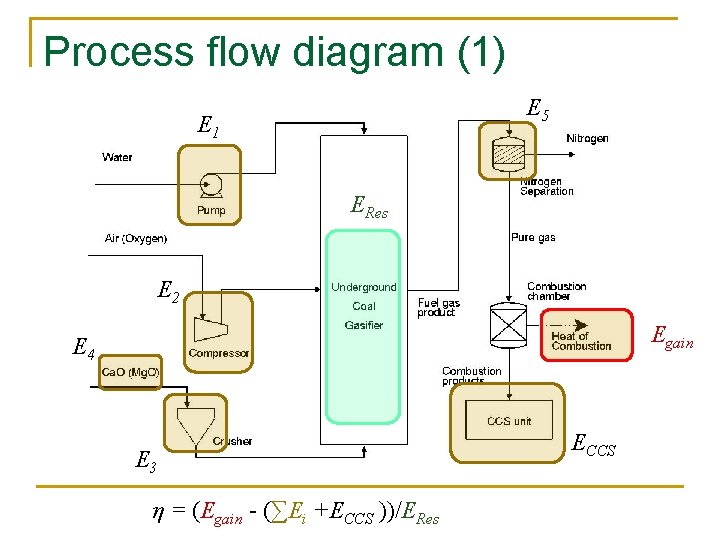
Process flow diagram (1) E 5 E 1 ERes E 2 Egain E 4 E 3 η = (Egain - (∑Ei +ECCS ))/ERes ECCS

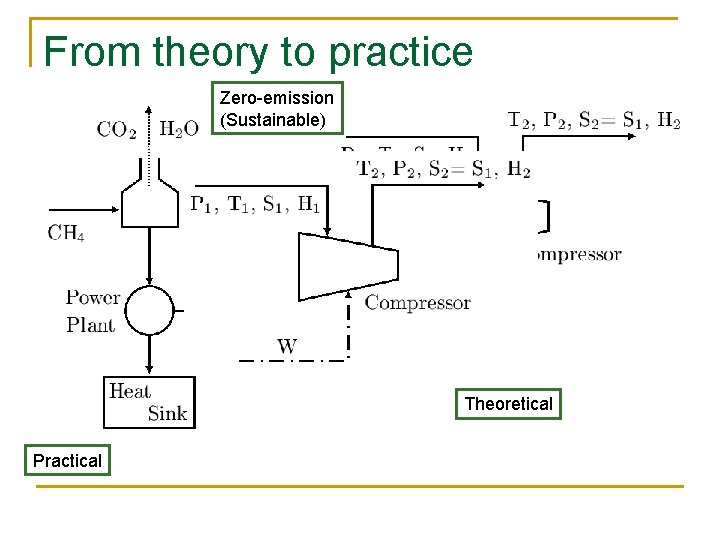
From theory to practice Zero-emission (Sustainable) Theoretical Practical

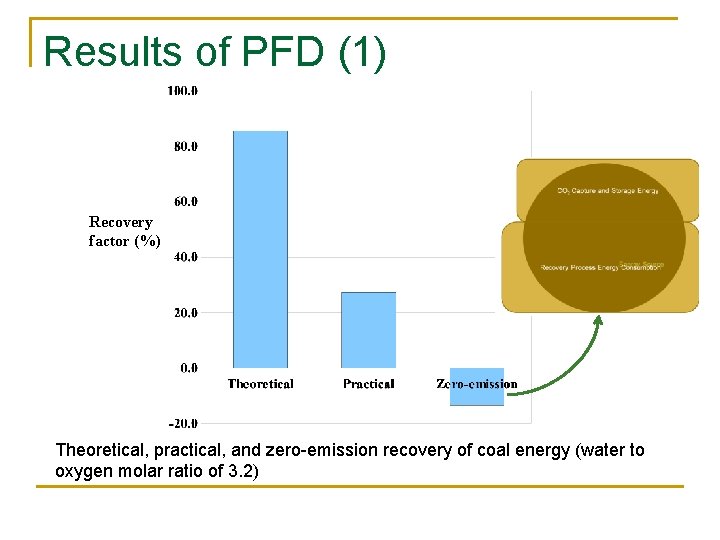
Results of PFD (1) Recovery factor (%) Theoretical, practical, and zero-emission recovery of coal energy (water to oxygen molar ratio of 3. 2)

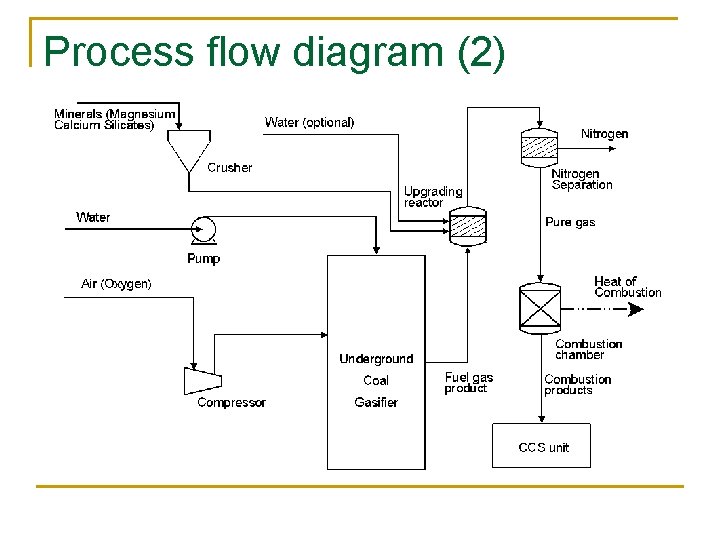
Process flow diagram (2)

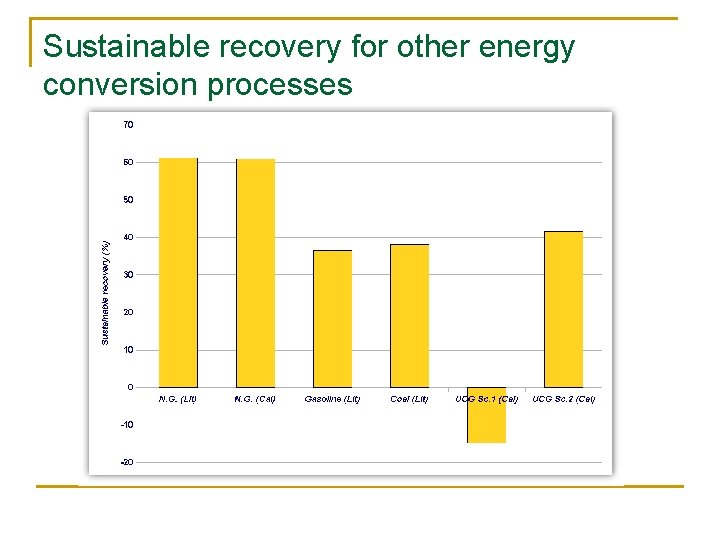
Sustainable recovery for other energy conversion processes

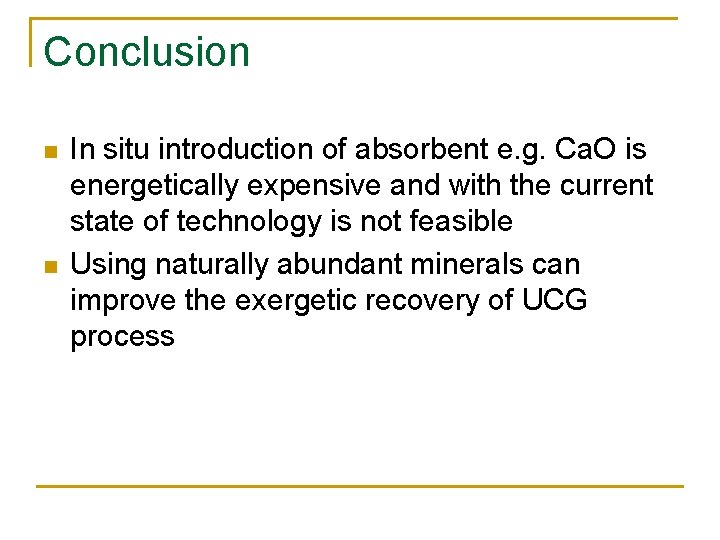
Conclusion n n In situ introduction of absorbent e. g. Ca. O is energetically expensive and with the current state of technology is not feasible Using naturally abundant minerals can improve the exergetic recovery of UCG process

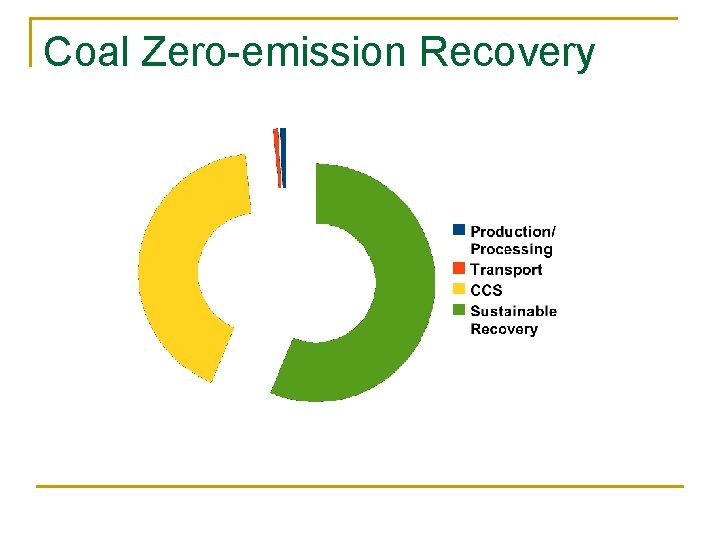
Coal Zero-emission Recovery

Natural gas sustainable recovery

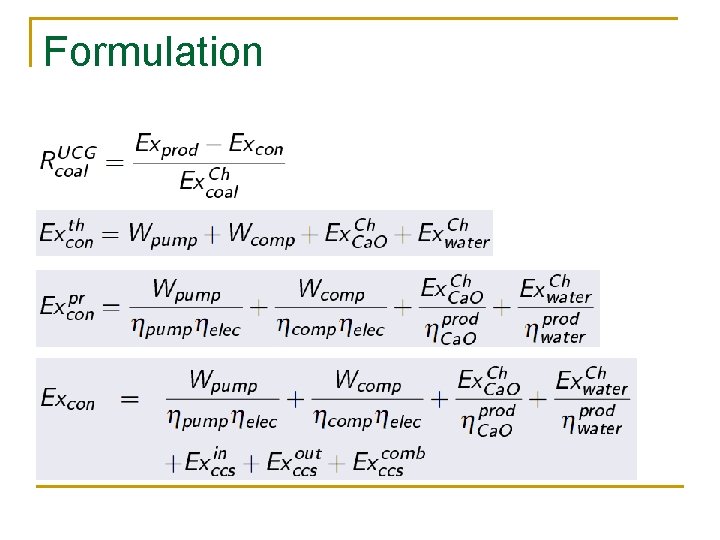
Formulation


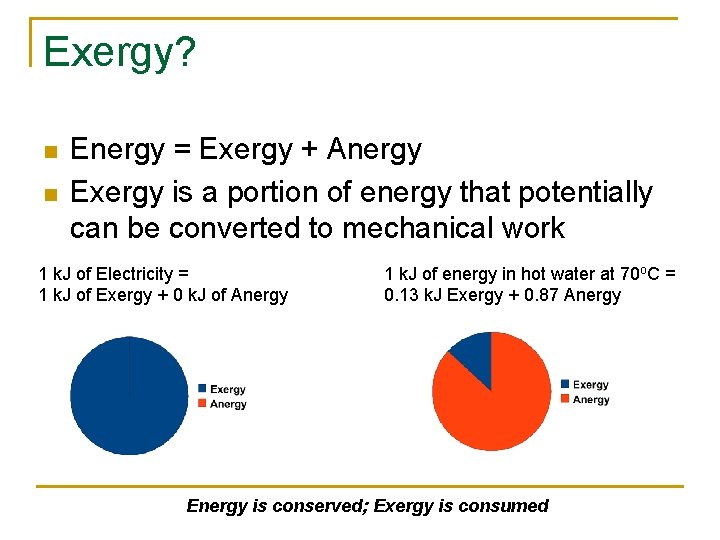
Exergy? n n Energy = Exergy + Anergy Exergy is a portion of energy that potentially can be converted to mechanical work 1 k. J of Electricity = 1 k. J of Exergy + 0 k. J of Anergy 1 k. J of energy in hot water at 70 o. C = 0. 13 k. J Exergy + 0. 87 Anergy Energy is conserved; Exergy is consumed
 Underground coal gasification
Underground coal gasification Plasma gasification
Plasma gasification Black liquor recovery boiler thermal spray
Black liquor recovery boiler thermal spray Starpower srl
Starpower srl Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Seamus heaney the underground
Seamus heaney the underground An abundance of coal an irregular coastline
An abundance of coal an irregular coastline Where does coal form
Where does coal form Cons of coal
Cons of coal Coal mining process
Coal mining process A ___ resource cannot be remade once it is used.
A ___ resource cannot be remade once it is used. Naphthalene chemical name
Naphthalene chemical name What is the composition of coal
What is the composition of coal Pros about coal
Pros about coal Stages of coal formation
Stages of coal formation Coal is the most abundant fossil fuel
Coal is the most abundant fossil fuel Children working in coal mines
Children working in coal mines Ashmasters chimney service inc
Ashmasters chimney service inc Cons of coal
Cons of coal Alex coal pmv
Alex coal pmv